Electrolyte for lithium metal battery and lithium metal battery thereof
A lithium metal battery and electrolyte technology, applied in the field of lithium metal battery electrolyte and lithium metal battery, can solve the problems of difficulty in meeting commercialization requirements, reduced electrochemical performance of lithium metal batteries at room temperature, poor film-forming effect, and the like, Achieve the effect of improving low temperature cycle performance, reducing lithium salt content, and low viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be further described below with reference to the embodiments and accompanying drawings, so that those skilled in the art can better understand the present invention and implement it, but the examples are not intended to limit the present invention.
[0020] The raw materials used in the following examples are all commercially available products and can be purchased.
[0021] Preparation of electrolyte:
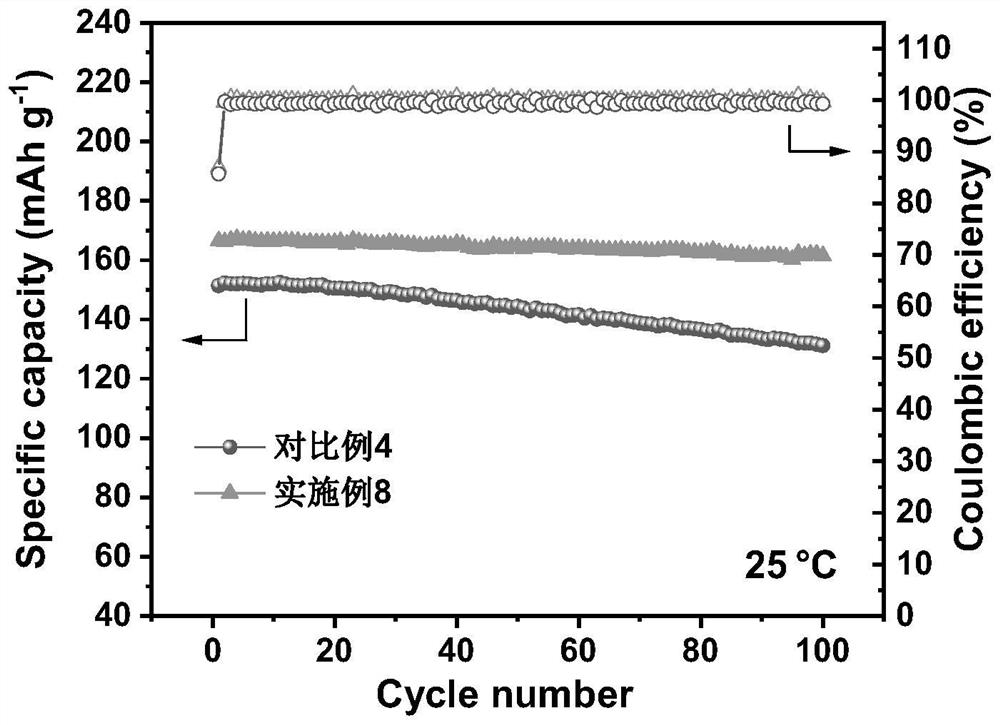
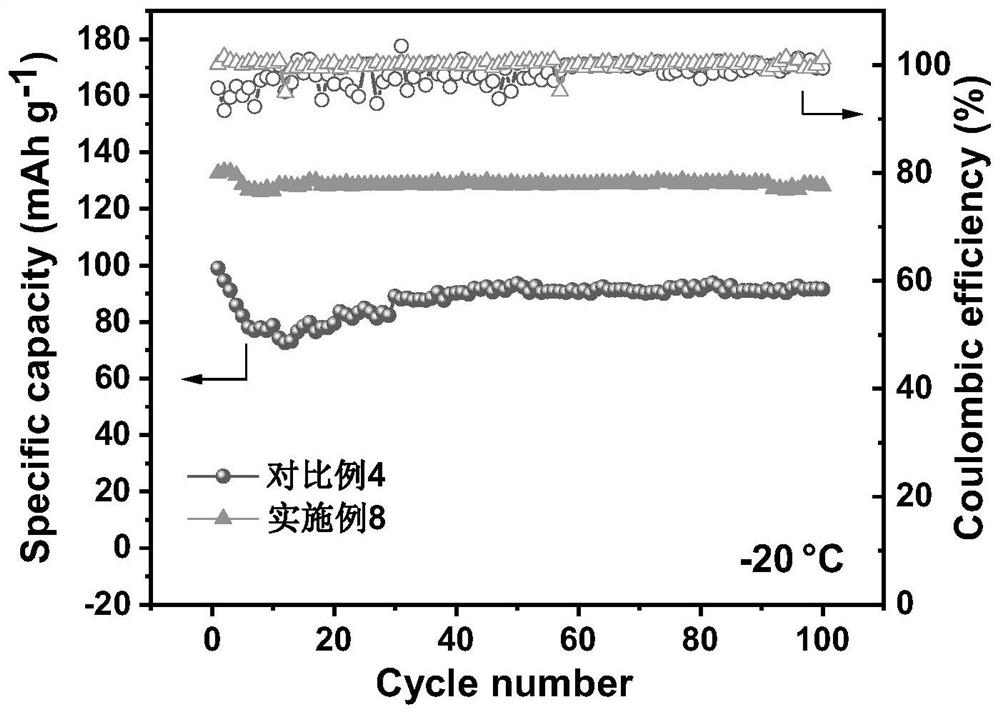
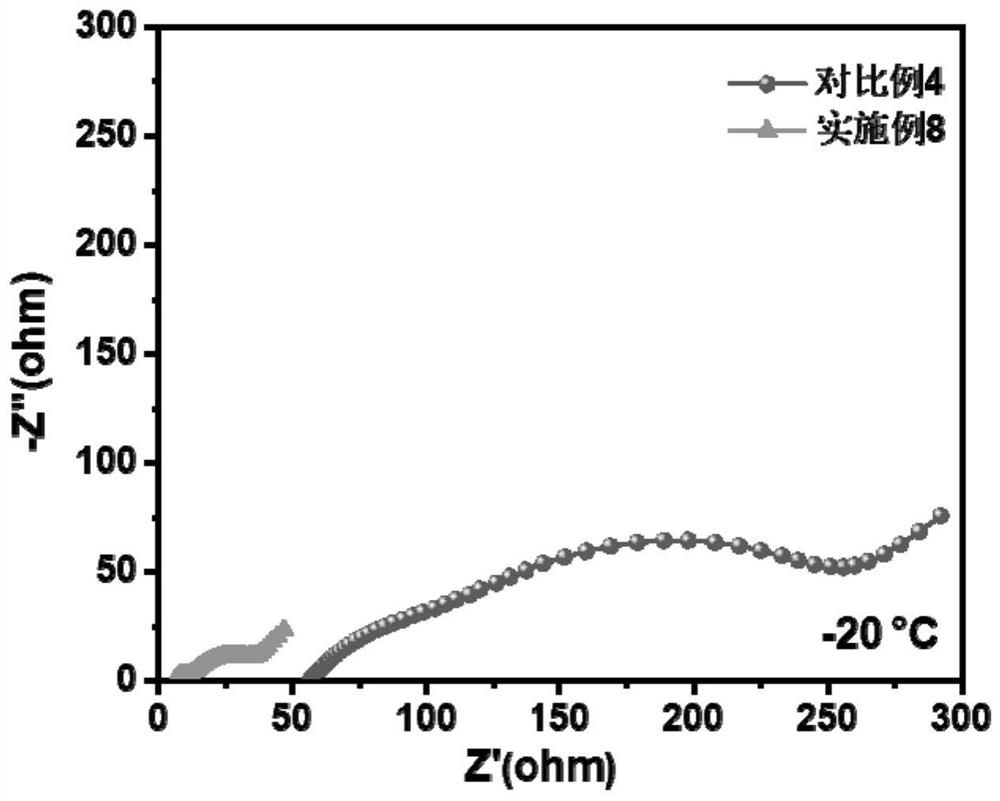
[0022] The electrolyte was prepared in a glove box with water and oxygen content less than 0.1 ppm, and the temperature was room temperature. According to the amount of raw materials in Table 1, the mixed lithium salt was added to the mixed solvent, fully mixed, and then allowed to stand for 24h for later use. Among them: GBL, PP, EP and FEC are γ-butyrolactone, propyl propionate, ethyl propionate and fluoroethylene carbonate respectively; LiBF 4 , LiPO 2 F 2 and LiPF 6 They are lithium tetrafluoroborate, lithium difluorophosphate and lit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com