Nuclide-labeled trastuzumab and preparation method thereof
A trastuzumab and labeling technology, applied in the field of clinical nuclear medicine, to achieve good targeting effect, inhibit and destroy diseased tissue, and achieve individualized effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
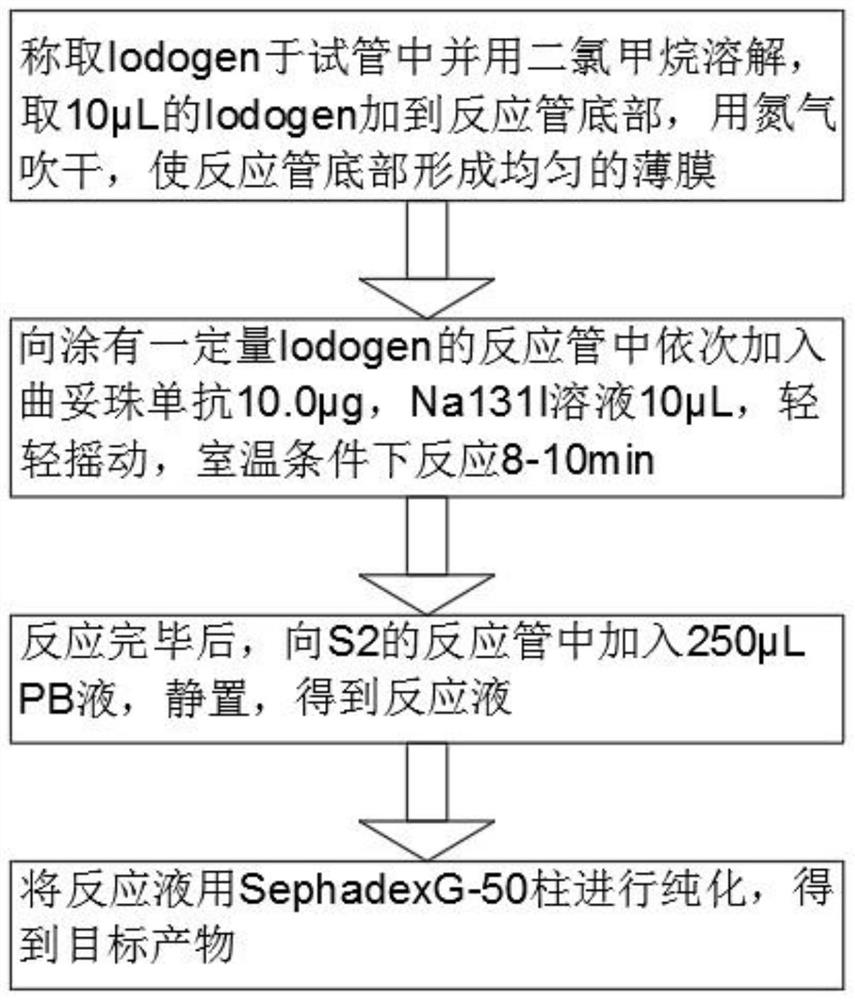
[0029] A radionuclide-labeled trastuzumab, which is a radionuclide 131I-labeled trastuzumab.
[0030] A preparation method of radionuclide-labeled trastuzumab, comprising the following steps:
[0031] S1: Weigh Iodogen in a test tube and dissolve it in dichloromethane, add 10 μL of Iodogen to the bottom of the reaction tube, and dry it with nitrogen to form a uniform film at the bottom of the reaction tube, and the concentration of dichloromethane is 1 g / L;
[0032] S2: Add 10.0 μg of trastuzumab and 10 μL of Na131I solution to the reaction tube coated with a certain amount of Iodogen in turn, shake gently, and react at room temperature for 8-10 minutes. The radioactivity of the Na131I solution is 22.2MBq;
[0033] S3: After the reaction is completed, add 250 μL of PB solution to the reaction tube of S2, and let it stand to obtain a reaction solution. The concentration of the PB solution is 0.05mol / L, and the standstill time is 5-8min;
[0034] S4: Purify the reaction solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radioactivity | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com