Method for simply preparing boron-nitrogen pyridine compound
A technology of borazine and compound, which is applied in the field of synthesizing borazine compounds, and achieves the effects of less by-products, low production cost and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
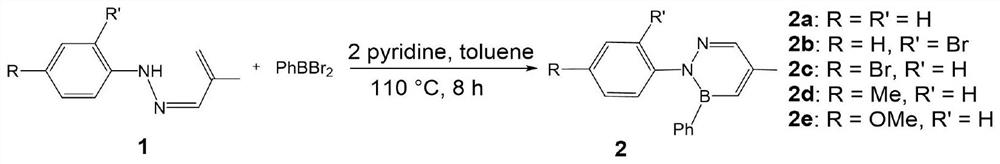
[0014] Add 108.1 mg of phenylhydrazine, 70.1 mg of 2-methacrolein, 2 drops of glacial acetic acid and 40 mL of ethanol into a 100-mL round-bottomed flask equipped with a magnet, and heated at 85 °C for 3-6 h. After the reaction, the reaction solution was spin-dried , and the aryl hydrazone was obtained by column separation and purification. In a glove box, 247.7 mg of phenylboron dibromide and 15 mL of toluene were added to a 50 mL Shrek bottle containing a magnet and mixed well. 160.2 mg of the aryl hydrazone synthesized above, 158.2 mg of pyridine and 40 mL of toluene were added to a 100 mL Shrek bottle, and a dropping funnel device was installed. Transfer the toluene solution of phenylboron dibromide to a dropping funnel, slowly add dropwise to the mixture of aryl hydrazone and pyridine at 0 °C, slowly return to room temperature, install a heating reflux device, and fill it with argon. balloon connection. The reaction was heated at 110 °C for 8 hours. After the reaction w...
Embodiment 2
[0019] 223.5mg of 2-bromophenylhydrazine hydrochloride, 101.2mg of triethylamine, 70.1mg of 2-methacrolein, 2 drops of glacial acetic acid and 40mL of ethanol were added to a 100mL round-bottomed flask with a magnet, heated at 85°C for 3 -6h, after the reaction, the reaction solution was spin-dried, and the aryl hydrazone was obtained by column separation and purification. In a glove box, add 247.7 mg of phenylboron dibromide and 15 mL of toluene into a 50 mL Shrek bottle with a magnet and mix well. 239.1 mg of the aryl hydrazone synthesized above, 158.2 mg of pyridine and 40 mL of toluene were added to a 100 mL Shrek bottle, and a dropping funnel device was installed. Transfer the toluene solution of phenylboron dibromide to a dropping funnel, slowly add dropwise to the mixture of aryl hydrazone and pyridine at 0 °C, slowly return to room temperature, install a heating reflux device, and fill it with argon. balloon connection. The reaction was heated at 110° C. for 8 h. Aft...
Embodiment 3
[0023] 223.5mg of 4-bromophenylhydrazine hydrochloride, 101.2mg of triethylamine, 70.1mg of 2-methacrolein, 2 drops of glacial acetic acid and 40mL of ethanol were added to a 100mL round-bottomed flask with magnetron, heated at 85°C 3-6h, after the reaction, the reaction solution was rotated to dryness, and the aryl hydrazone was obtained by column separation and purification. In a glove box, add 247.7 mg of phenylboron dibromide and 15 mL of toluene into a 50 mL Shrek bottle with a magnet and mix well. 239.1 mg of the aryl hydrazone synthesized above, 158.2 mg of pyridine and 40 mL of toluene were added to a 100 mL Shrek bottle, and a dropping funnel device was installed. Transfer the toluene solution of phenylboron dibromide to a dropping funnel, slowly add dropwise to the mixture of aryl hydrazone and pyridine at 0 °C, slowly return to room temperature, install a heating reflux device, and fill it with argon. balloon connection. The reaction was heated at 110° C. for 8 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com