Preparation method of 1-(tert-butyl)-3-chloronaphthalene
A technology of chloronaphthalene and naphthylamine, which is applied in the field of preparation of 1--3-chloronaphthalene, can solve the problems of many by-products, low yield of target product, complicated process, etc., and achieve less by-products, low cost and environmental friendliness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
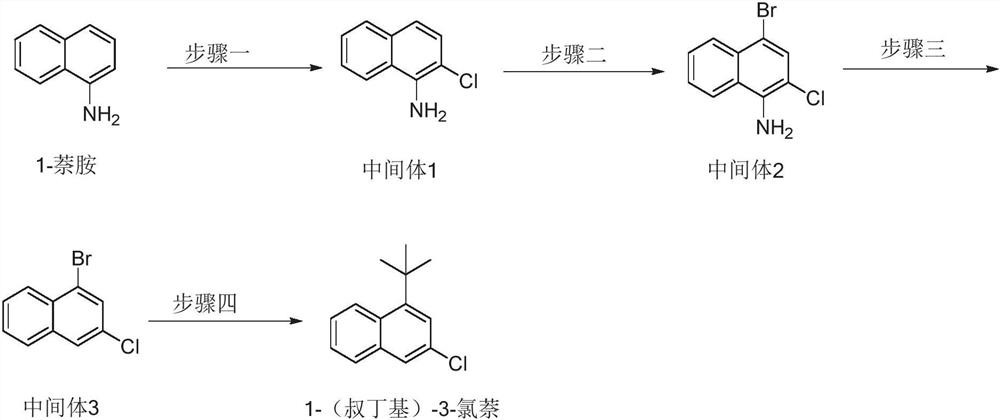
[0039] Further, a kind of preparation method of 1-(tert-butyl)-3-chloronaphthalene in this typical embodiment comprises the following steps:
[0040] S1. Dissolving 1-naphthylamine in a solvent, adding N-chlorosuccinimide, and extracting after completion of the reaction to obtain intermediate 1 (ie, the aforementioned first intermediate).
[0041] S2. Dissolving intermediate 1 in a solvent, adding N-bromosuccinimide, and extracting after completion of the reaction to obtain intermediate 2 (ie, the aforementioned second intermediate).
[0042] S3. Dissolving intermediate 2 in a solvent, adding Lewis acid and tert-butyl nitrite, and stirring for a certain period of time, adding hypophosphorous acid and cuprous oxide. After completion of the reaction, extraction and column chromatography were performed to obtain Intermediate 3 (ie, the aforementioned third intermediate).
[0043] S4, adding catalyst ligand, nickel acetylacetonate and lithium tert-butoxide into tetrahydrofuran, a...
Embodiment 1
[0046] A preparation method of 1-(tert-butyl)-3-chloronaphthalene comprises the following steps:
[0047] S1. Weigh 1-naphthylamine (about 69.8 mmol), dissolve it in tetrahydrofuran (about 200 mL), slowly add N-chlorosuccinimide (about 69.8 mmol) at 0 °C, and stir at 0 °C for 1 hour. After the reaction was completed, dichloromethane and water were added to the reaction solution, and the layers were extracted and separated. The organic phase was washed twice with saturated sodium carbonate, then washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain Intermediate 1 (about 7.8 g). , the yield is 63%). Characterization results of the intermediate 11HNMR (400MHz, CDCl3): δ=7.83-7.79(m, 2H), 7.54-7.47(m, 2H), 7.54-7.47(m, 2H), 7.38(d, 1H, J = 8.0 Hz), 7.26 (d, 1H, J = 8.0 Hz), 4.58 (brs, 2H).
[0048] S2. Weigh intermediate 1 (about 43.9 mmol), dissolve it in dichloromethane (about 100 mL), add N-bromosuccinimide (about 43.9 mmol) at 0 °C, and...
Embodiment 2
[0052] The preparation method of a kind of 1-(tert-butyl)-3-chloronaphthalene provided by this embodiment comprises the following steps:
[0053] S1, the molar ratio of 1-naphthylamine and N-chlorosuccinimide was adjusted to 1:0.8, the reaction was carried out at 2°C, and the rest were the same as step S1 in Example 1.
[0054] S2. The molar ratio of the first intermediate to the brominating reagent is adjusted to 1:1.2, the brominating reagent is liquid bromine, the reaction is carried out at 2°C, and the rest are the same as in step S2 of Example 1.
[0055] The molar ratio of S3, the second intermediate, Lewis acid, alkyl nitrite, reducing agent, and the first catalyst is adjusted to 1:3:1.2:20:0.05, and the Lewis acid adopts p-toluenesulfonic acid and alkane nitrite. Isoamyl nitrite was used as the base ester, the reaction was carried out at 2°C, and the rest were the same as in step S3 of Example 1.
[0056] S4, the molar ratio of the catalyst ligand, the second catalyst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com