Carbon monoxide production method and application thereof
A production method and carbon monoxide technology, applied in the furnace, blast furnace details, furnace type and other directions, can solve the problems of unpredictable use of reducing agent, low separation and recovery rate, and low economical hydrogen reduction technology, so as to reduce the cost of reducing agent and reduce The effect of emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Comparison based on carbon monoxide conversion
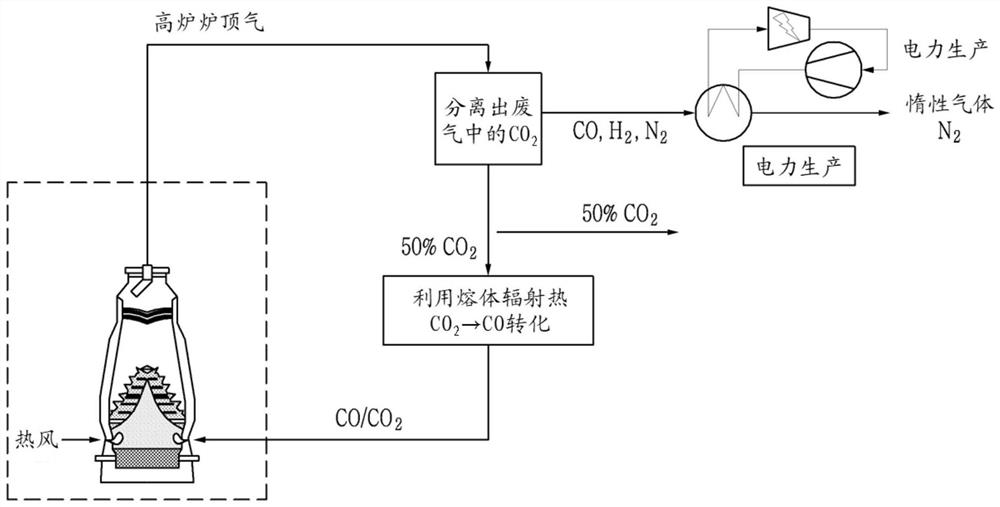
[0073]In the present invention, the carbon dioxide gas in the blast furnace waste gas is reformed into carbon monoxide, then re-injected through the blast furnace tuyere, and calculated by the thermal material balance equation to understand the blast furnace heat balance and carbon dioxide emission reduction effect. Table 1 shows the equilibrium and carbon dioxide emission reduction effects in the blast furnace caused by re-blowing carbon monoxide to the blast furnace tuyere according to the carbon monoxide gas conversion rate prepared according to an embodiment of the present invention for molten iron.
[0074] 【Table 1】
[0075]
[0076] *Base represents an existing blast furnace into which reformed carbon monoxide is not injected.
[0077] *t-p means ton-pig (ton pig iron).
[0078] [Formula 3] Pulverized coal blowing amount based on CO conversion rate (kg / ton pig iron)=-0.007*[CO conversion rate (%)] ...
Embodiment 2
[0081] Example 2: CO gas re-injection based on carbon monoxide conversion
[0082] The amount of CO gas that is re-blown into the blast furnace according to the carbon dioxide conversion rate is known through experiments.
[0083] The CO gas conversion rate was changed as shown in Table 2 below, and the amount of waste gas and molten iron production was kept constant.
[0084] The CO gas re-injection amount was calculated according to the following formula 1.
[0085] [Formula 1] Re-injection amount of CO gas (Nm 3 / min) = CO2 gas content in exhaust gas (Nm 3 / ton) × molten iron production (ton / day) × CO2 gas ratio (50%) × CO gas conversion rate (%) × (1 day / 1440 minutes)
[0086] 【Table 2】
[0087]
[0088]
[0089] From the results shown in the above table, it can be seen that when the CO gas re-injection amount is greater than 1270Nm 3 At the time of / min, the amount of carbon dioxide blown back into the blast furnace is increased, and therefore, the amount of...
Embodiment 3
[0090] Example 3: Comparison of specific surface areas of carbon-containing by-products
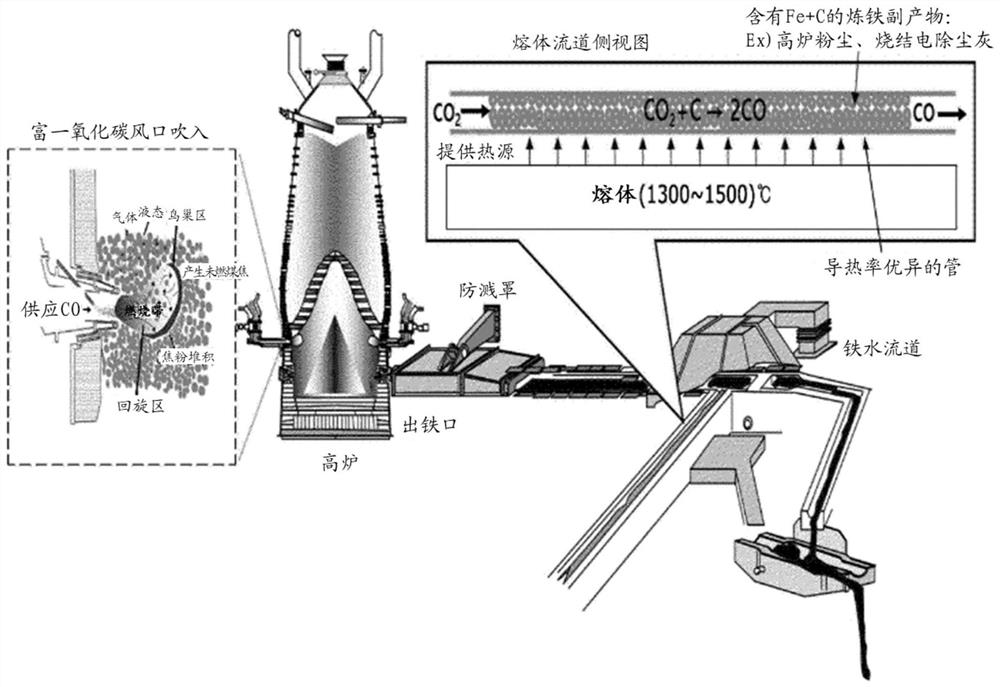
[0091] In order to realize the production of carbon monoxide in a carbon dioxide reformer using radiant heat in the flow channel, carbon dioxide was blown into reaction tubes filled with carbon-containing by-products with different specific surface areas as shown in Table 3, respectively, and the conversion rate according to the temperature was measured. , and the results are shown in Table 3. It can be seen that as the reaction temperature becomes higher and higher due to the radiant heat of the flow channel, the conversion of carbon monoxide increases, and at the same reaction temperature, a 20% higher conversion of carbon-containing by-products with good specific surface area is observed (see image 3 ). However, re-injecting carbon monoxide with a conversion rate of 85% means that the total input gas blown into the blast furnace will decrease, so it is necessary to increase the oxyge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com