Ginsenoside docetaxel liposome as well as preparation method and application thereof
A technology of ginsenoside and docetaxel, which is applied in the directions of liposome delivery, pharmaceutical formulations, and medical preparations of inactive ingredients, etc., can solve the problems of not giving the relationship between pharmacokinetics and toxicology, and achieve good medicines. Synergistic effect, reduced side effects, improved Glut1 targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
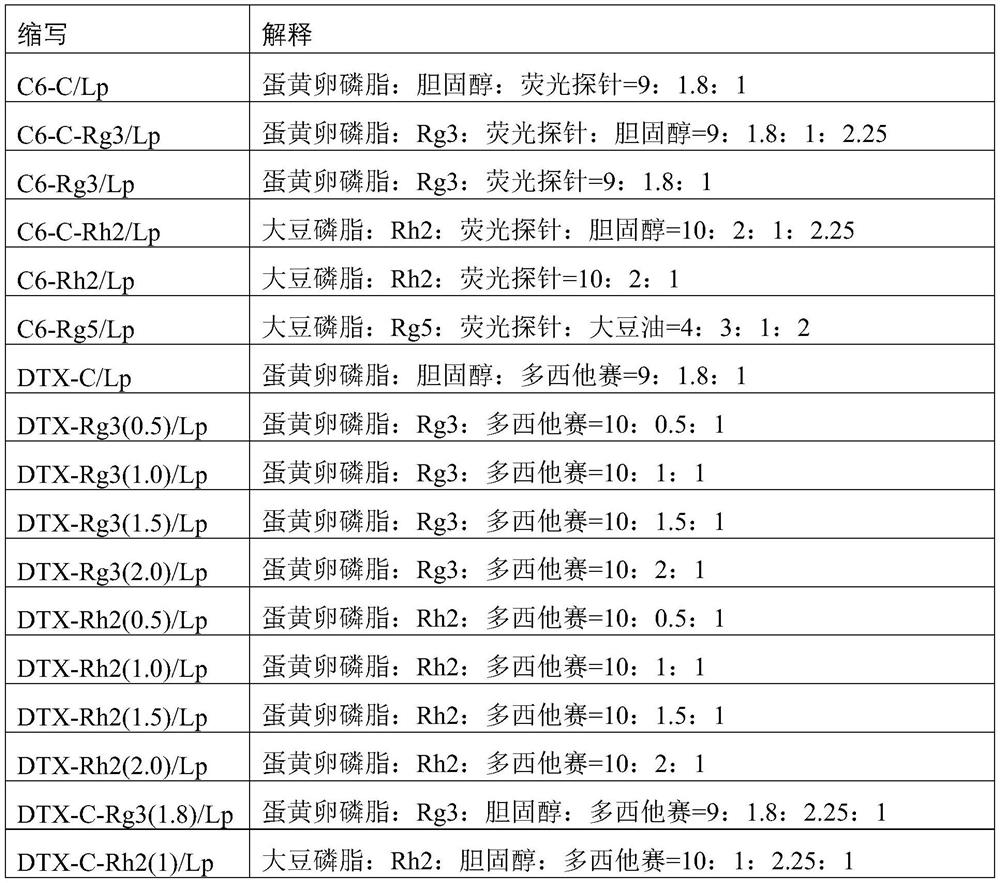
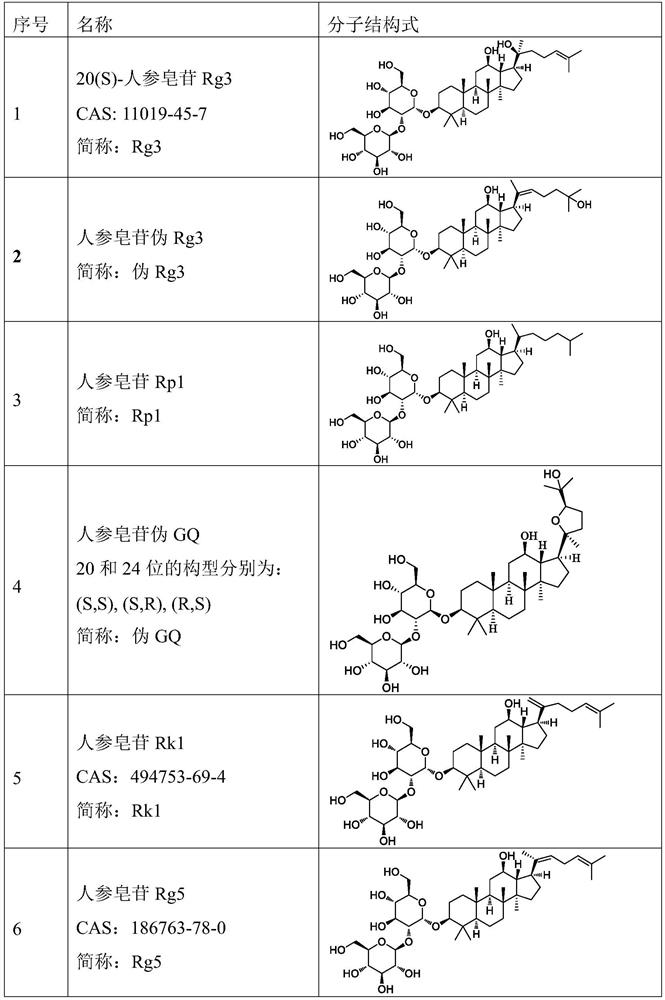
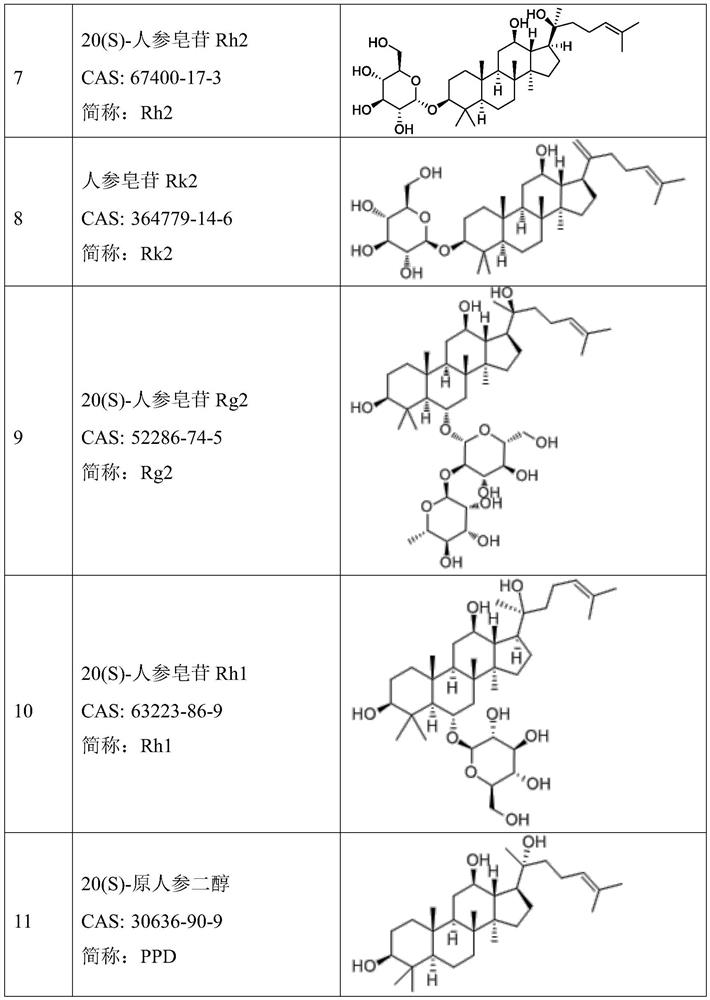
[0150] Example 1 Preparation of compound ginsenoside Rg3 docetaxel liposome for injection
[0151] 1. Prescription: egg yolk lecithin 10g, ginsenoside Rg3 1g, docetaxel 1g, glucose 25g, absolute ethanol 40ml, chloroform 40ml, water for injection 200ml.
[0152] 2. Film formation: prepare a mixed solvent of absolute ethanol and chloroform (1:1) in the prescribed amount for use.
[0153] Add the prescription amount of docetaxel to the mixed solvent to dissolve for later use, then add the prescription amount of ginsenoside Rg3 and egg yolk lecithin into the mixed solvent, heat to dissolve, transfer into a 1L rotary steamer, concentrate under reduced pressure, and the water bath temperature is 55 ° C , rotating speed 50 rev / min, vacuum degree -0.089~-0.1MPa, rotary steam until all the solvent is completely volatilized.
[0154] 3. Hydration: Preparation of glucose solution: Add 25g of anhydrous glucose to 100ml of water for injection, stir and dissolve, and then prepare a 0.25mg / ...
Embodiment 2
[0164] Example 2 Preparation of Compound Ginsenoside Rg3 Docetaxel Liposomes for Injection
[0165] The prescription amount of the ginsenoside Rg3 in Example 1 was increased to 1.5 g, and the others were the same as in Example 1, and the compound ginsenoside Rg3 docetaxel liposome for injection was prepared.
Embodiment 3
[0166] Example 3 Preparation of Compound Ginsenoside Rg3 Docetaxel Liposomes for Injection
[0167] 1. Prescription: egg yolk lecithin 10g, ginsenoside Rg3 1.5g, docetaxel 1g, glucose 25g, absolute ethanol 40ml, chloroform 40ml, water for injection 200ml.
[0168] 2. Film formation: the same as the film formation method of Example 1.
[0169] 3. Hydration: the same as the hydration method of Example 1.
[0170] 4. High-speed shearing and extrusion: the above-mentioned liposome solution was rapidly sheared at 2000 rp / min for 5 min at room temperature.
[0171] The temperature of the liposome solution was controlled at 35-45 °C, an extrusion device was connected, an extrusion plate with a 150 nm aperture was installed, and the extrusion was performed under a pressure of 800 psi.
[0172] 5. Subsequent steps are the same as those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com