Preparation method and application of environment-friendly polyurethane catalyst
A polyurethane catalyst and an environment-friendly technology, applied in the field of preparation of an environment-friendly polyurethane catalyst, can solve the problems of restricting the development of polyurethane and human injury, and achieve the effects of improving yield, simple operation and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
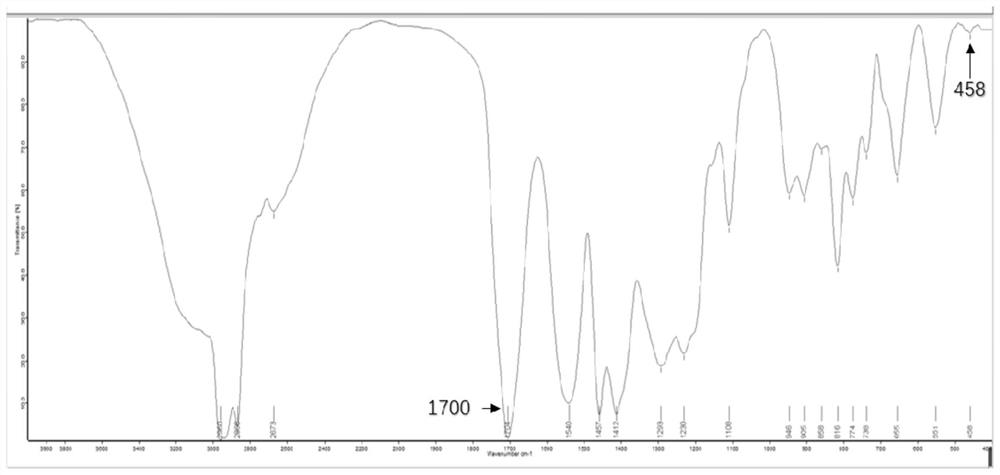
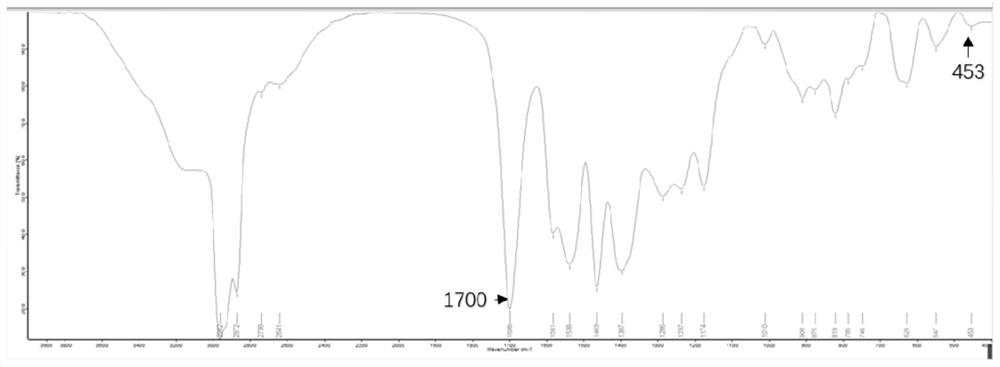
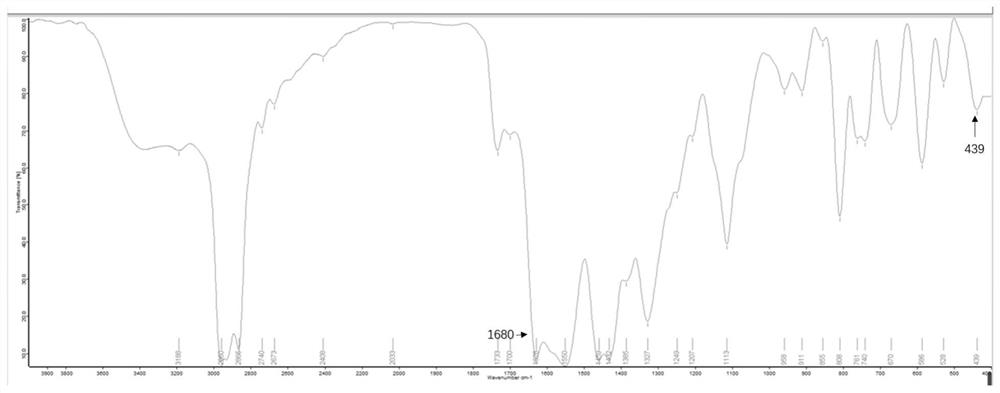
Image
Examples
Embodiment 1
[0036] (1) 0.81g of zinc oxide is dissolved in 200mL of 0.1mol / L dilute hydrochloric acid, and the pH of the solution is adjusted to 3 with dilute hydrochloric acid to obtain an environmentally friendly organic zinc solution;
[0037] (2) 21.6g of tridecanoic acid was dissolved in 100mL of 1mol / L sodium hydroxide solution, heated to 70°C, maintained for 3 hours, and the solid was dissolved to obtain a carboxylate solution;
[0038] (3) adding 100mL environment-friendly organic zinc solution and 100mL valeric acid to the 100ml carboxylate solution obtained in step (2), stirring evenly, heating to 80° C., and reacting for 2 hours to obtain a product solution that generates precipitation; Cooling, vacuum filtration with a Buchner funnel, and repeatedly rinsed with 60°C distilled water for 6 times; the washed product is placed in a 120°C vacuum drying oven for drying to obtain an environmentally friendly organozinc catalyst.
[0039] The catalyst yield obtained in this example was...
Embodiment 2
[0042] (1) 1.36g of zinc chloride is dissolved in 200mL of 0.1mol / L dilute nitric acid, and the pH of the solution is adjusted to be 4 with dilute nitric acid to obtain an environmentally friendly organic zinc solution;
[0043] (2) 14.4g of isooctanoic acid was dissolved in 100mL of 1mol / L sodium hydroxide solution, heated to 60°C, maintained for 3 hours, and the solid was dissolved to obtain a carboxylate solution;
[0044] (3) adding 100mL environment-friendly organic zinc solution and 100mL mineral oil to the 100ml carboxylate solution obtained in step (2), stirring evenly, heating to 70° C., and reacting for 3 hours to obtain a viscous product solution; Cool the solution, use a separating funnel to stand for stratification, separate the water phase, perform vacuum distillation on the organic phase to obtain the product, and repeatedly rinse the product with 50°C distilled water for 8 times; place the washed product in a 100°C vacuum drying oven. Drying is carried out in t...
Embodiment 3
[0048] (1) 1.89g of zinc nitrate was dissolved in 200mL of 0.1mol / L dilute nitric acid, and the pH of the solution was adjusted to 3 with dilute nitric acid to obtain an environmentally friendly organic zinc solution;
[0049] (2) 17.2g of neodecanoic acid was dissolved in 100mL of 1mol / L sodium hydroxide solution, heated to 70°C, maintained for 3 hours, and the solid was dissolved to obtain a carboxylate solution;
[0050](3) in the 100ml carboxylate solution obtained in step (2), add environment-friendly 100mL organic zinc solution and 150mL propionic acid, stir, be heated to 60 ℃, react 4 hours, obtain the product solution that generates precipitation;
[0051] The product solution was cooled, vacuum filtered with a Buchner funnel, and washed with distilled water at 60°C for 7 times; the washed product was placed in a vacuum drying oven at 100°C for drying to obtain an environmentally friendly organozinc catalyst.
[0052] The catalyst yield obtained in this example was 76....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com