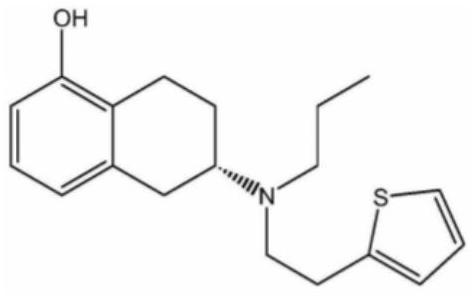
Rotigotine transdermal absorption patch
A rotigotine, transdermal technology, applied in the directions of drug combination, nervous system diseases, organic active ingredients, etc., can solve the problem that rotigotine is prone to crystallization, the release degree of rotigotine is reduced, and the Transdermal absorption patch is unstable and other problems, to achieve the effect of not easy to fall off, increase adhesion, and ensure quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] composition Dosage (g) rotigotine 9 Silicone pressure sensitive adhesive 4301 65 Silicone pressure sensitive adhesive 4201 43 Lauryl Lactate 3 Povidone 4 ascorbyl palmitate 0.02 Tocopherol 0.05 Sodium metabisulfite 0.0006
[0030] Preparation:
[0031] (1) dissolving sodium metabisulfite in purified water to obtain 10% sodium metabisulfite solution; dissolving 10% sodium metabisulfite solution, ascorbyl palmitate, tocopherol, povidone and lauryl lactate in ethanol to obtain an auxiliary material solution, spare;
[0032] (2) adding rotigotine to the auxiliary material solution obtained in step (1), stirring and dissolving completely, and then stirring with silicone pressure-sensitive adhesive 4301 and silicone pressure-sensitive adhesive 4201 to obtain a drug-containing colloidal solution;
[0033] (3) Coating the drug-containing colloid solution obtained in step (2) with a coating machine, and cutting to o...
Embodiment 2
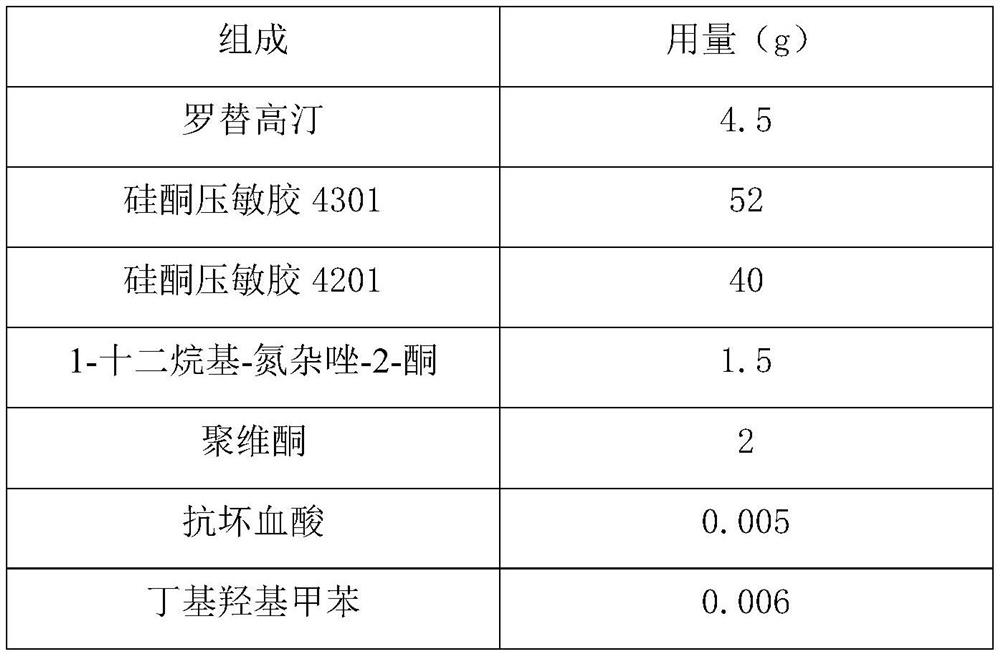
[0035]
[0036]
[0037] Preparation:
[0038] (1) ascorbic acid, butylated hydroxytoluene, propyl gallate, povidone and 1-dodecyl-azazol-2-one are dissolved in ethanol to obtain auxiliary material solution, for subsequent use;
[0039] (2) heating the auxiliary material solution obtained in step (1), adding rotigotine when the temperature is 30-40 ℃, heating up to 50-60 ℃, adding silicone pressure-sensitive adhesive 4301 and silicone pressure-sensitive adhesive 4201, The rotation speed is 40-100RPM for uniform stirring, and then the rotation speed is 1500-3000RPM for uniform stirring to obtain a drug-containing colloid solution;
[0040] (3) Coating the drug-containing colloid solution obtained in step (2) with a coating machine, and cutting to obtain it.
Embodiment 3
[0042] composition Dosage (g) rotigotine 10 Silicone pressure sensitive adhesive 4301 46 Silicone pressure sensitive adhesive 4201 34 Lauryl Lactate 2 Menthol heptyl ester 2 Povidone 5.5 Butyl Hydroxyanisole 0.35 Vitamin E 0.15
[0043] Preparation:
[0044] (1) butylated hydroxyanisole, vitamin E, povidone, lauryl lactate and menthol heptyl ester are dissolved in ethanol to obtain auxiliary material solution, for subsequent use;
[0045](2) heating the auxiliary material solution obtained in step (1), adding rotigotine when the temperature is 30-40 ℃, heating up to 50-60 ℃, adding silicone pressure-sensitive adhesive 4301 and silicone pressure-sensitive adhesive 4201, The rotation speed is 40-100RPM for uniform stirring, and then the rotation speed is 1500-3000RPM for uniform stirring to obtain a drug-containing colloid solution;
[0046] (3) Coating the drug-containing colloid solution obtained in step (2) with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com