Anti-SIRP alpha monoclonal antibody
A monoclonal antibody and antigen technology, applied in the direction of antibodies, anti-tumor drugs, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problems of reducing immune response and adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Example 1 Acquisition of anti-SIRPα antibody
[0186] (1) Prepare antigen, the concrete steps are as follows:
[0187] a. Obtain the target gene
[0188] In this example, the protein sequence of SIRPα (Uniprot: P78324) was searched from Uniprot, and amino acids 31-373 were selected, and the SIRPα extracellular region was connected with the mouse heavy chain constant region through three linking peptides (GGGGS). Sequence optimization and DNA synthesis were performed by GenScript Biotechnology Co., Ltd. The optimally synthesized PUC-57-SIRPa-mFC plasmid and PGS plasmid were digested with HindIII and EcoRI, and the target fragment was recovered by agarose gel recovery kit, and ligated with T4 DNA ligase at 16°C overnight.
[0189] b. Construction of recombinant eukaryotic expression vector
[0190] Both the sequence and the vector PGS were digested with restriction enzymes HindIII and EcoRI, and the target fragment was recovered and ligated with T4 ligase to construct ...
Embodiment 2
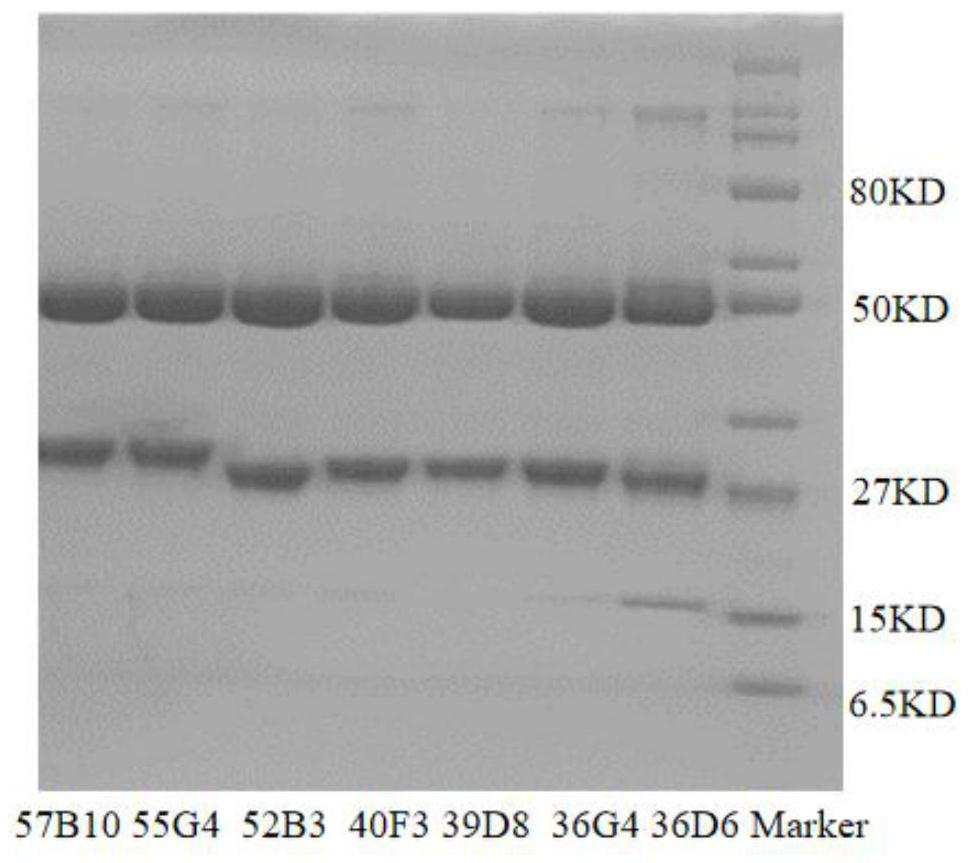
[0226] Example 2 Detection of Antibody Molecular Weight by SDS-PAGE
[0227] SDS-PAGE electrophoresis was carried out according to the method in the third appendix of Chinese Pharmacopoeia to identify its molecular weight and expression. The SDS-PAGE pattern of the purified antibody is as follows figure 1 As shown, the molecular weights are about 50kd and 25kd respectively, which are in line with the molecular weights of the antibody heavy chain and light chain, and the purity is above 95%.
Embodiment 3
[0228] Example 3 Variable region amplification of anti-SIRPα murine monoclonal antibody
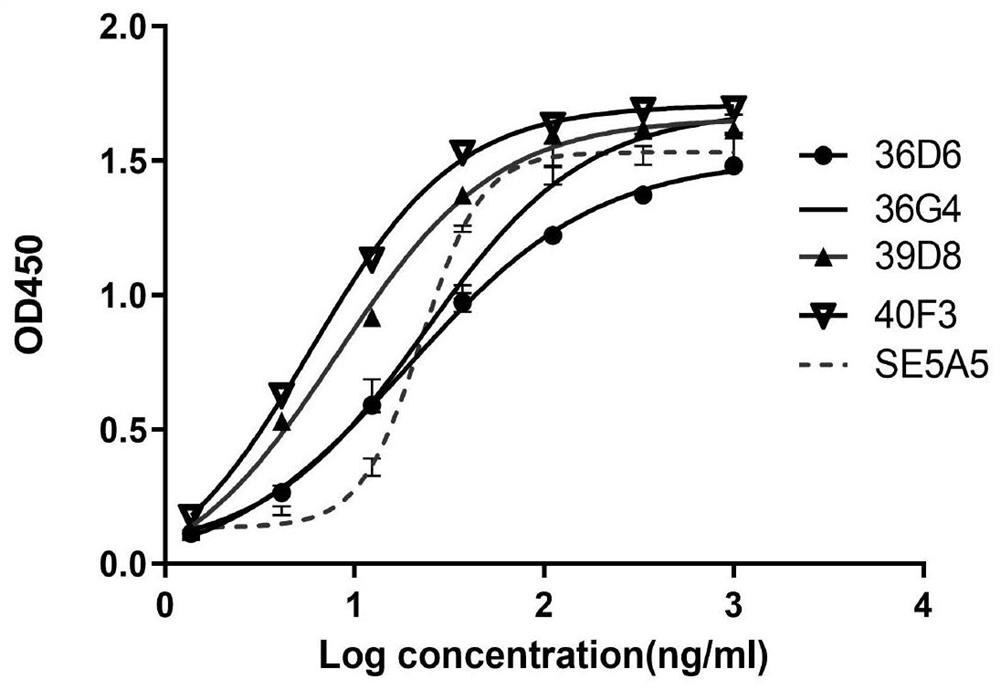
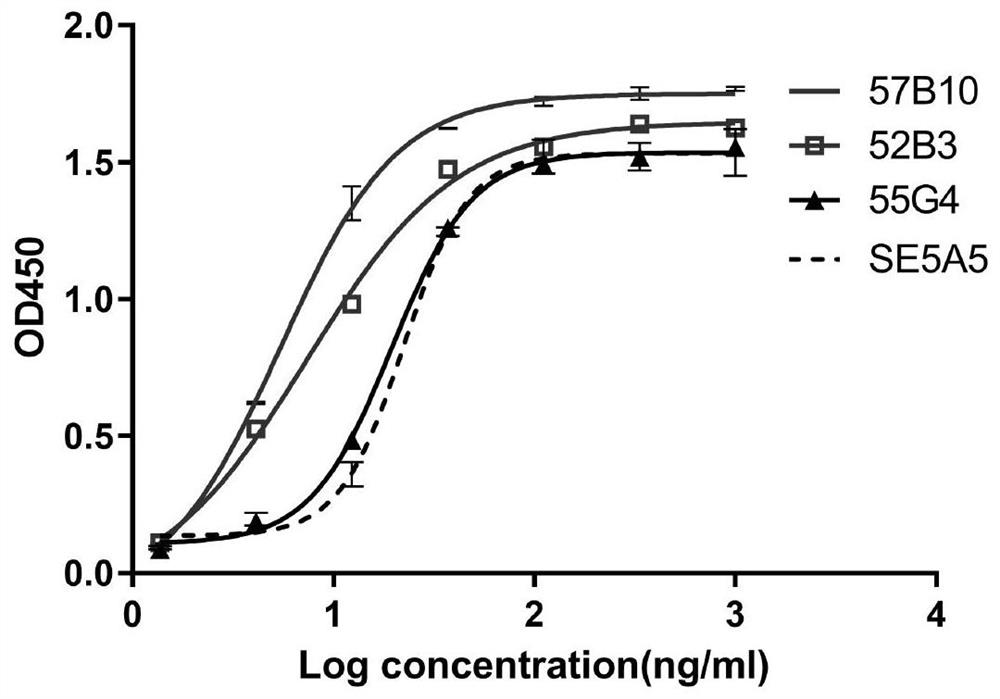
[0229] The candidate hybridoma cells 36D6, 36G4, 39D8, 40F3, 52B3, 55G4, and 57B10 were cultured to logarithmic growth phase, and the cells were collected by centrifugation at 1000 rpm for 10 min. box prime script TM The first-strand cDNA was synthesized by RT-PCR, and the DNA sequence of the antibody variable region corresponding to the hybridoma cells was amplified by using the first-strand cDNA as the subsequent template. According to the subtype identification results, the heavy chain and light chain constant region sequences of the antibody subtype are obtained, involving specific nested PCR primers. The primer sequences used in the amplification reaction are the same as the antibody variable region first framework region and antibody variable region. Complementary constant regions.
[0230] (1) Cloning of heavy chain variable regions of murine antibodies 36D6, 36G4, 39D8, 40F3, 52...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com