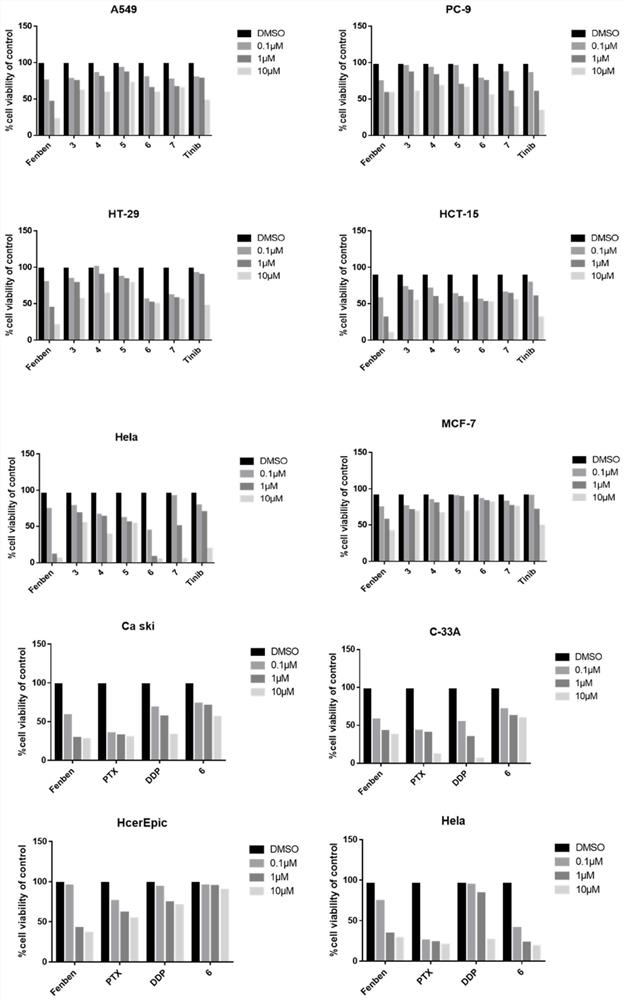
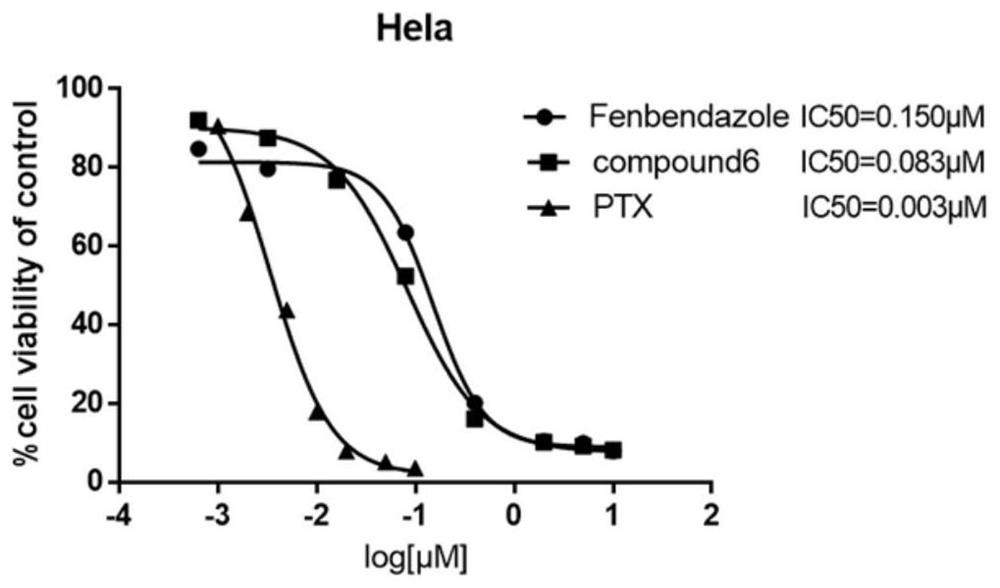
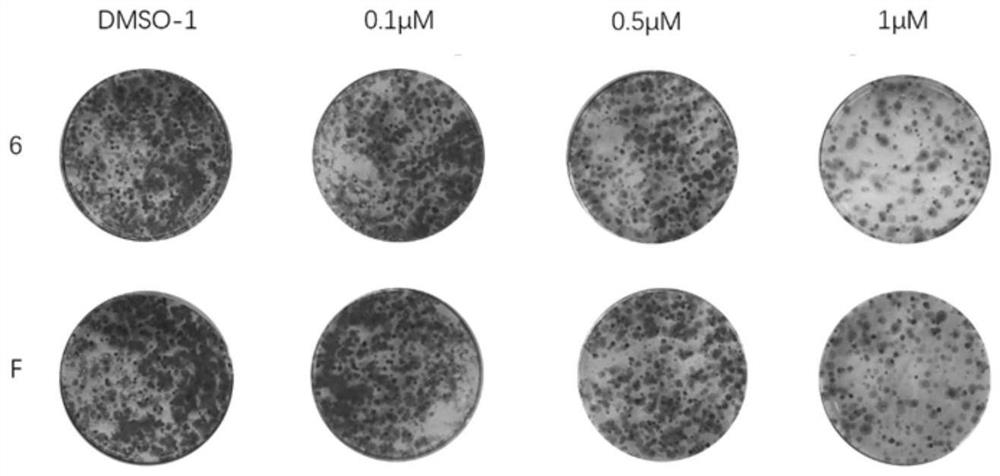
Fenbendazole analogue as well as preparation method and application thereof
A technology of fenbendazole and analogues, applied in the field of fenbendazole analogues and its preparation, to achieve the effects of inhibiting colony formation, inhibiting migration and invasion, inducing apoptosis and cell cycle arrest
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 (preparation of compound 3)
[0050]
[0051] Compound 3a (3.0 g, 12.2 mmol) was added to a mixed solution of EtOH (80.0 mL) / THF (30 mL) at 25°C, followed by Pd / C (3.0 g). Put the reaction mixture in H 2 The mixture was stirred at 25°C for 3h. The reaction mixture was then filtered and vacuum filtered to give 3b (2.5 g, 96.1%) as a black oil.
[0052] To a solution of 3b (2.5 g, 11.6 mmol) in DMF (30.0 mL) at 25 °C was added 3c (3.2 g, 13.9 mmol) and 4-methyl-morpholine (3.8 mL, 34.8 mmol). The reaction mixture was heated under nitrogen (N 2 ) at 25°C for 5 min, then EDCI.HCl (2.9 g, 15.1 mmol) and HOBt (2.0 g, 15.1 mmol) were added. Put the reaction mixture in N 2 Stir under protection at 25°C for 3h. mixture with H 2 Diluted with 0 (50 mL) and extracted with ethyl acetate (100 mL). The organic layer was washed with brine (100 mL) and concentrated. The crude product was purified by silica gel chromatography (EA:PE=1:2) to give 3d and 3e (4.8 g, 97...
Embodiment 2
[0056] Example 2 (preparation of compound 4)
[0057]
[0058] To a solution of compound 4a (5.0 g, 18.3 mmol) and MsCl (5.5 g, 27.5 mmol) in THF (300 mL) at 0 °C was added TEA (13 mL, 91.5 mmol). The reaction mixture was stirred at 70 °C for 16 h. The organic phase was dried and concentrated in vacuo and the residue was purified by column chromatography (eluting with PE in EA 0-90% to give 4d (5.1 g, yield: 19%) 4e (3.2 g, yield: 19%) .
[0059] To a solution of 4c (23.0 g, 117.3 mmol) in DMF (400 mL) was added NaH (7.0 g, 176.0 mmol) at 0 °C, and the reaction solution was stirred at 25 °C for 1 h. 4b (35.0 g, 125.4 mmol) was added dropwise to the mixture at 0°C. The reaction solution was stirred at 25 °C for 16 h, poured into ice water (1000 mL), the organic phase was dried, concentrated in vacuo, and the residue was purified by column chromatography (eluted with PE in EA 0-90% to give 4d (5.1 g, Yield: 19%) 4e (3.2 g, Yield: 19%).
[0060] 4d (3.2g, 8.41mmol), 4f (1...
Embodiment 3
[0062] Example 3 (preparation of compound 5)
[0063]
[0064] 4e (3.2g, 8.41mmol), Pd(dppf)Cl 2 (616.0 mg, 0.84 mmol), Xant-phos (486.4 mg, 0.84 mmol), t-BuOK (1.9 g, 16.82 mmol) and 4f (2.2 mmol, 16.82 mmol) in dioxane (30 mL) at 100 °C argon Stir under gas protection for 16h. The mixture was filtered and washed with ethyl acetate (50 mL). The filtrate was concentrated in vacuo and purified by silica gel chromatography (PE:ethyl acetate=5:1) to give 5a (2.9 g, yield: 85%) as a yellow solid.
[0065] Compound 5a (2.9 g, 7.08 mmol) was combined with a solution of HCl-EtOAc (3.0 M, 30 mL). Stir at 10°C for 3h. The resulting reaction mixture was concentrated in vacuo to give 5b (2.7 g, yield: >99%).
[0066] To hydrochloride-5b (2.79 g, 8.07 mmol) in THF / H 2 Add K to O (20mL / 5mL) solution 3PO4 (8.57 g, 40.35 mmol). The mixture was stirred at 10 °C for 5 min. 5 mL of acryloyl chloride in THF (803.37 mg, 8.88 mmol) was added slowly. The reaction mixture was stirred at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com