Method for synthesizing decitabine
A synthetic method, the technology of decitabine, applied in the field of medicinal chemistry, can solve the problems of not increasing the ratio of β-configuration products of glycosylation reaction, poor selectivity of glycosylation reaction, and low yield of β-isomer, and achieve cost Low, easy to separate, short preparation route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
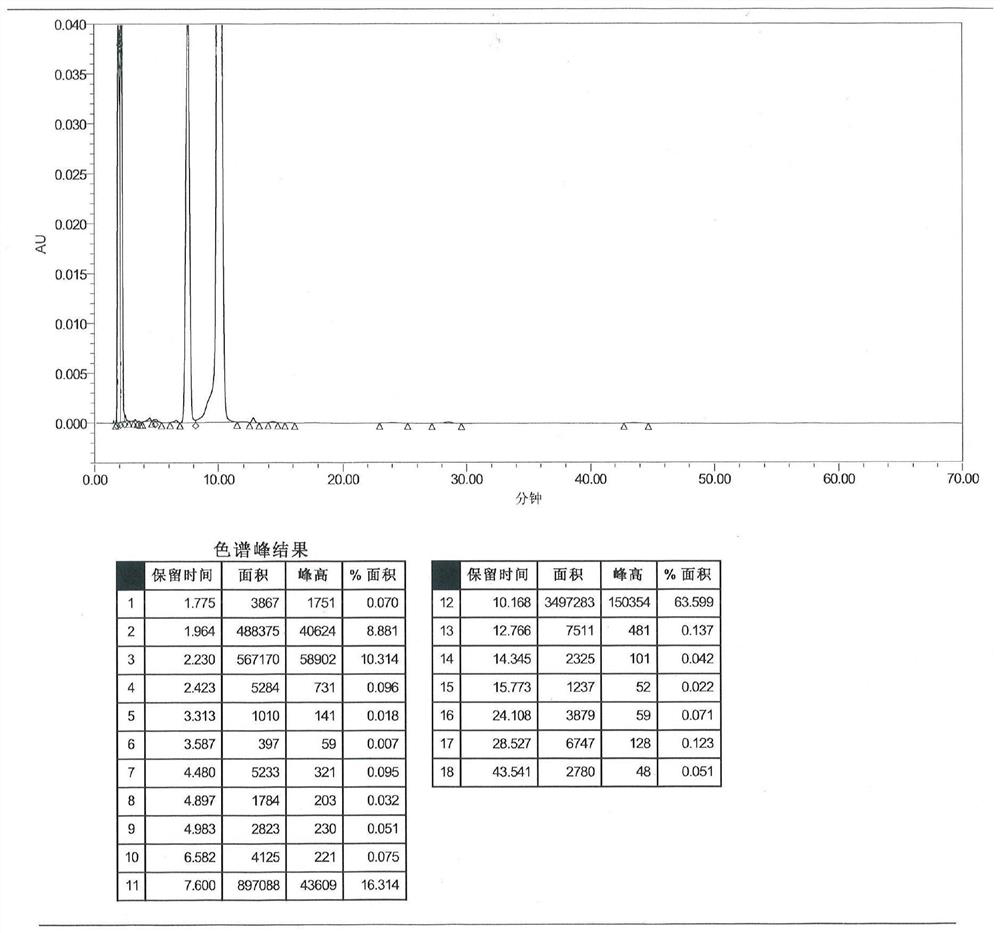
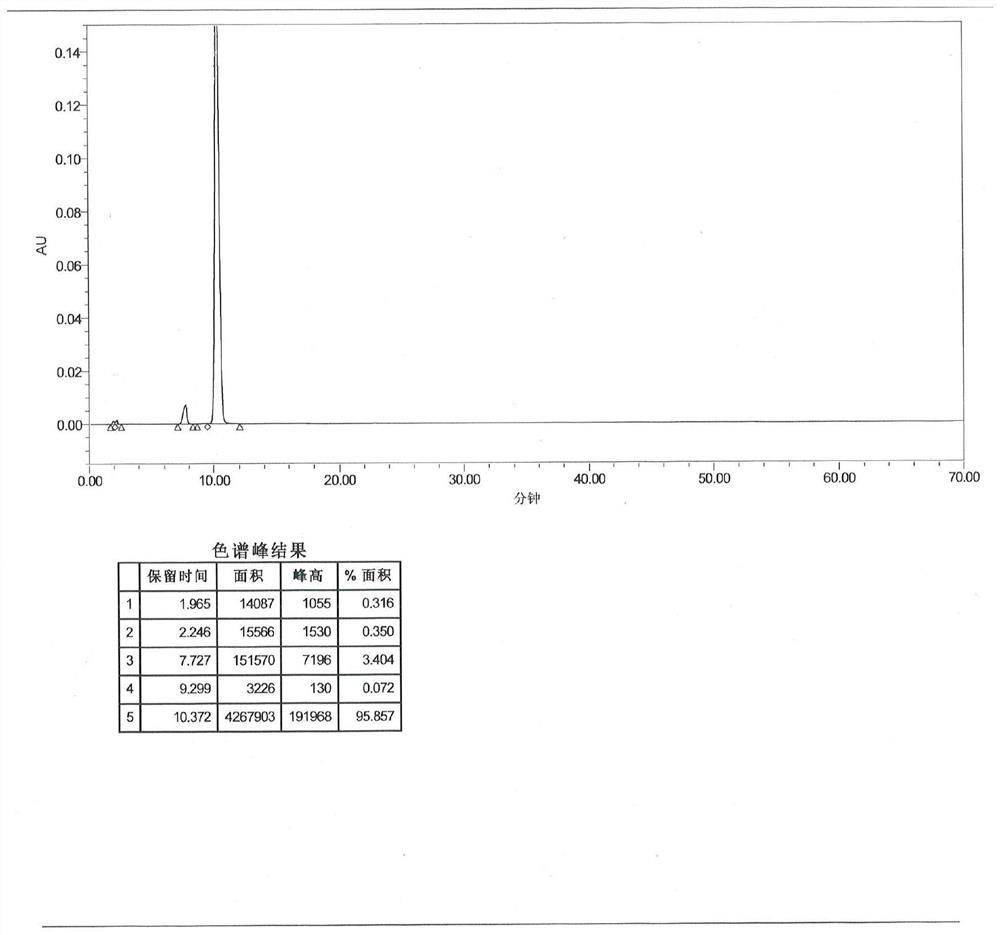
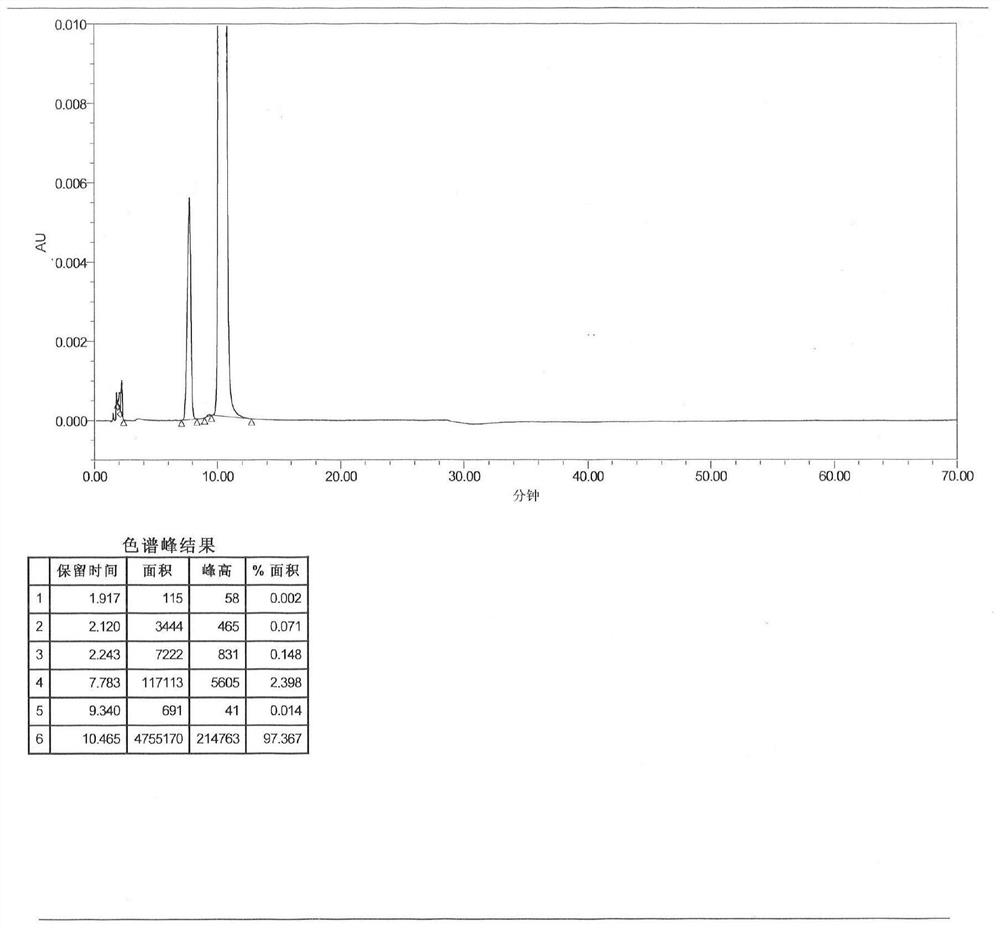
Image
Examples
Embodiment 1
[0048] Add 50 g of compound II to the reaction flask, add 750 mL of chloroform and 56 g of pyridine, cool down to 5 °C, add 195 g of tert-butyldiphenylchlorosilane dropwise, keep stirring for 2 h after the drop, add 750 mL of sodium phosphate buffer solution , the liquid is separated, the organic phase is dried with 50 g of anhydrous sodium sulfate, and filtered to obtain the chloroform solution of compound II-1.
[0049]The chloroform solution of compound II-1 was added to the reaction flask, 86.48 g of compound III was added, the temperature was lowered to 10 °C, and 88 g of tin tetrachloride was added dropwise. : ethyl acetate=4:1) detected the basic reaction was completed, the reaction solution was slowly added to 4L saturated sodium bicarbonate solution, the liquid was separated, and the organic phase was evaporated to dryness under reduced pressure to obtain the colorless oily substance of compound IV (α and β different The composition ratio is about 1:4, see for details...
Embodiment 2
[0052] Add 50 g of compound II to the reaction flask, add 500 mL of chloroform and 53 g of pyridine, cool down to 5 °C, add 185 g of tert-butyldiphenylchlorosilane dropwise, keep stirring for 2 h after the drop, add 500 mL of sodium phosphate buffer solution , the liquid is separated, the organic phase is dried with 50 g of anhydrous sodium sulfate, and filtered to obtain the chloroform solution of compound II-1.
[0053] The chloroform solution of compound II-1 was added to the reaction flask, 95.13 g of compound III was added, the temperature was lowered to 10°C, 96.8 g of tin tetrachloride was added dropwise, the dropping was completed, and the reaction was kept under stirring for 6 to 8 hours. Ether: ethyl acetate=4:1) detected that the basic reaction was completed, the reaction solution was slowly added to 4L of saturated sodium bicarbonate solution, separated, and the organic phase was evaporated to dryness under reduced pressure to obtain a colorless oily substance (α an...
Embodiment 3
[0056] Add 50 g of compound II to the reaction flask, add 1 L of chloroform and 66.3 g of pyridine, cool down to 5 °C, add 232 g of tert-butyldiphenylchlorosilane dropwise, keep stirring for 2 h after the drop, add 750 mL of sodium phosphate buffer The liquid was separated, and the organic phase was dried with 50 g of anhydrous sodium sulfate, and filtered to obtain a chloroform solution of compound II-1.
[0057] The chloroform solution of compound II-1 was added to the reaction flask, 86.48 g of compound III was added, the temperature was lowered to 10 °C, and 88 g of tin tetrachloride was added dropwise. : ethyl acetate=4:1) detected the basic reaction was completed, the reaction solution was slowly added to 4L saturated sodium bicarbonate solution, the liquid was separated, and the organic phase was evaporated to dryness under reduced pressure to obtain the colorless oily substance of compound IV (α and β different The composition ratio is about 1:4). 150 mL of acetonitri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com