Application of glibenclamide composition in preparation of medicine for treating increase of brain water content
A technology of glibenclamide and its composition, which is applied in the field of pharmaceutical use, can solve problems such as complex pathophysiological mechanisms and no treatment plan, and achieve the effects of short operation time, simple operation, and reduction of brain water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
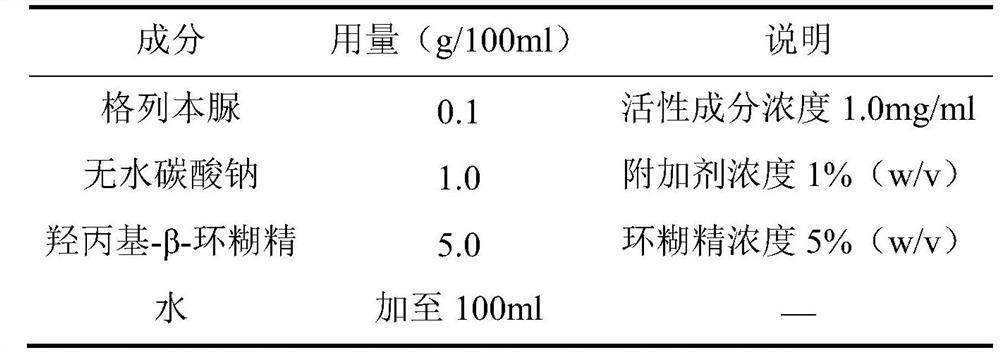
[0035] Example 1 Preparation of Glyburide Injection Composition (lyophilized powder for injection)
[0036] prescription:
[0037]
[0038] The preparation method is as follows: according to the prescription, dissolving anhydrous sodium carbonate and cyclodextrin in 50-70 ml of water, then adding glyburide powder, stirring and dissolving to obtain a first solution. A 2 mol / L hydrochloric acid solution was added to the first solution to adjust the pH to 8.0-8.5, and the volume was adjusted to 100 ml to obtain a second solution. The second solution is divided into vials, each vial of 3 ml, and freeze-drying is performed on the second solution in the vials to obtain freeze-dried powder injection. Freeze dry using the lyophilization procedure described in the table below.
[0039] Freeze drying procedure
[0040]
Embodiment 2
[0041] Example 2: Preparation of Glyburide Injection Composition (lyophilized powder for injection)
[0042] prescription:
[0043]
[0044] Preparation method: first dissolve anhydrous sodium carbonate and sulfobutyl ether-β-cyclodextrin in 80 ml of water, then add glyburide powder, stir and dissolve to obtain a first solution. 2 mol / L hydrochloric acid was added to the first solution to adjust the pH of the solution to 8.0-8.5, and the volume was adjusted to 100 ml to obtain a second solution. The second solution is divided into vials, each vial is 3 ml, and freeze-drying is performed on the second solution in the vials (the freeze-drying procedure is the same as that in Example 1) to obtain freeze-dried powder injection.
Embodiment 3
[0045] Example 3: Preparation of Glyburide Injection Composition (lyophilized powder for injection)
[0046] prescription:
[0047]
[0048] Preparation method: firstly dissolving meglumine and sulfobutyl ether-β-cyclodextrin in 80 ml of water, then adding glyburide powder, stirring and dissolving to obtain a first solution. 0.1M hydrochloric acid was added to the first solution to adjust the pH of the solution to 8.0-8.5, and the volume was adjusted to 100 ml to obtain a second solution. The second solution is divided into vials, each vial is 3 ml, and freeze-drying is performed on the second solution in the vials (the freeze-drying procedure is the same as that in Example 1) to obtain freeze-dried powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com