Primer probe composition and kit for detecting canine parainfluenza virus, canine adenovirus type II and canine mycoplasma and preparation method of primer probe composition
A technology of canine parainfluenza virus and canine adenovirus, which is applied in biochemical equipment and methods, recombinant DNA technology, microbial measurement/inspection, etc., can solve the problems of unfriendly pathogen identification, poor sensitivity, and low sensitivity, and achieve optimization Reaction system and procedures, avoiding false positive results, and improving the effect of detection throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 A primer probe of a nucleic acid detection kit for canine parainfluenza virus, canine adenovirus type II and Mycoplasma canis
[0024] The above primer probes were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
Embodiment 2
[0025] Example 2 Preparation method of nucleic acid detection kit for canine parainfluenza virus, canine adenovirus type II and Mycoplasma canis
Step 1. Prepare negative and positive controls
Negative control is TE Buffer;
Positive control: Insert the CPIV target gene sequence, CAV-II target gene sequence, and MC target gene sequence into the pUC 57 vector plasmid, respectively, and obtain CPIV synthetic plasmid, CAV-II synthetic plasmid, MC through expanded culture, identification and plasmid extraction. Artificially synthesized plasmids. The CPIV target gene sequence is shown in SEQ ID NO.13; the CAV-II target gene sequence is shown in SEQ ID NO.14; the MC target gene sequence is shown in SEQ ID NO.15.
[0026] The CPIV synthetic plasmid, CAV-II synthetic plasmid, and MC synthetic plasmid were quantified to 3 × 10, respectively. 6 After copy / mL, mix them according to the volume ratio of 1:1:1 to make a positive control. In the obtained positive control, the concentrat...
Embodiment 3
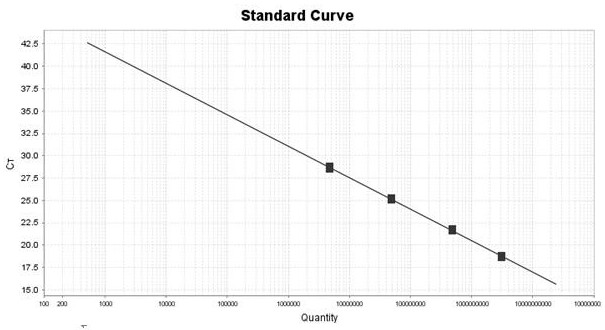
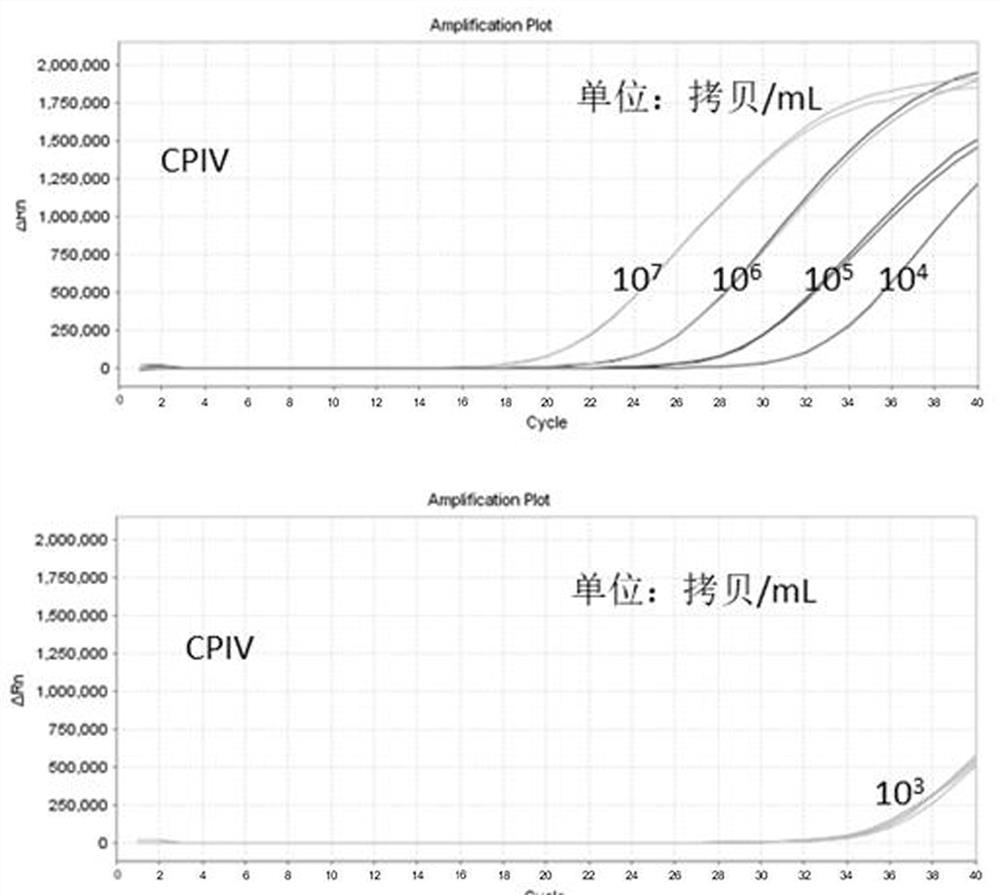
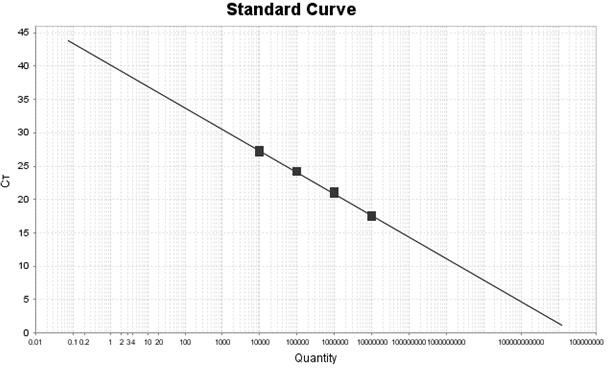
[0033] Example 3 Kit Linearity, Sensitivity, Repeatability and Specificity Detection Experiment
Step 1. Template preparation
Insert the CPIV target gene sequence, CAV-Ⅱ target gene sequence, and MC target gene sequence into the pUC 57 vector plasmid respectively, and obtain CPIV synthetic plasmid, CAV-Ⅱ synthetic plasmid and MC synthetic plasmid through expansion culture, identification and plasmid extraction. . The CPIV synthetic plasmid, CAV-II synthetic plasmid, and MC synthetic plasmid were quantified to 1 × 10, respectively. 7 copies / mL.
[0034] The CPIV target gene sequence is shown in SEQ ID NO.13; the CAV-II target gene sequence is shown in SEQ ID NO.14; the MC target gene sequence is shown in SEQ ID NO.15.
[0035] Will 1x10 7 The copies / mL of CPIV synthetic plasmid, CAV-II synthetic plasmid and MC synthetic plasmid were diluted to 1×10 with TE Buffer respectively. 6 Copy / mL, 1×10 5 Copy / mL, 1×10 4 Copy / mL, 1×10 3 Copy / mL, spare.
[0036] Take canine parvo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com