Application of fixed bed catalytic reactor in removal of hydrazine nitrate and hydroxylamine nitrate
A catalytic reactor and fixed bed technology, applied in the direction of chemical instruments and methods, nitric acid, nitrogen oxides/oxyacids, etc., can solve problems such as insufficient reaction, violent reaction, leakage, etc., and achieve no safety hazards and simple operation , the effect of excellent economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
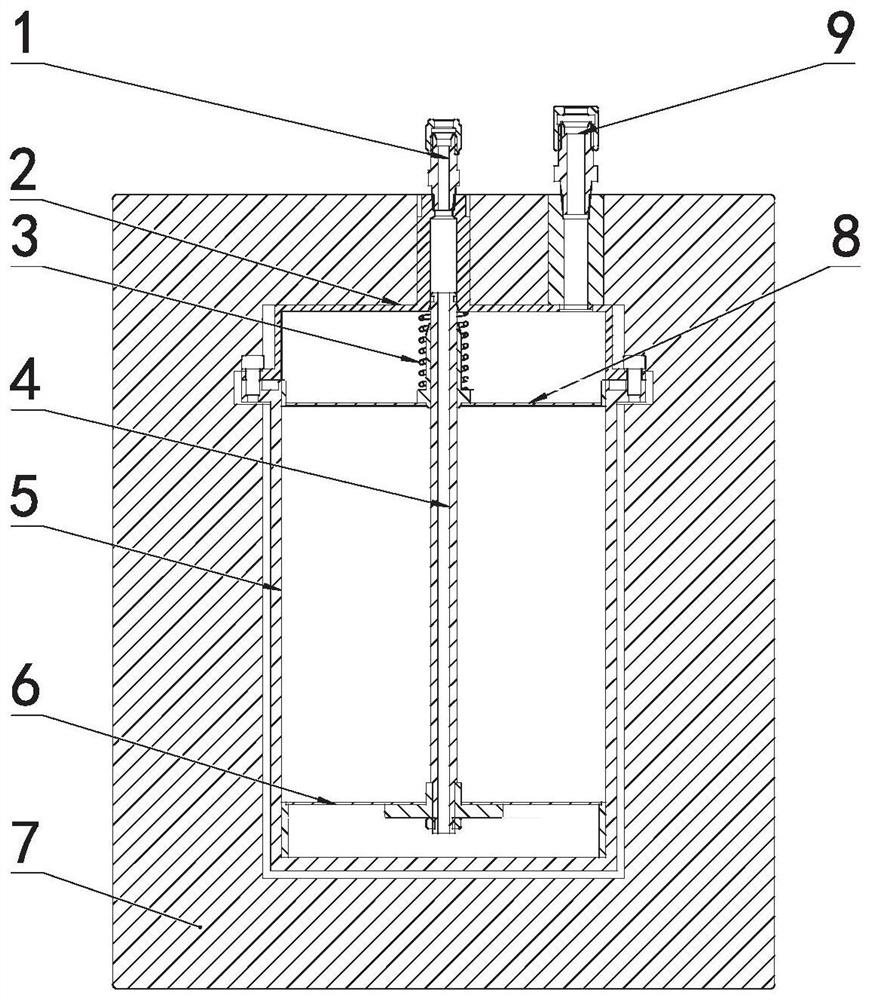
[0091] Weigh 700g of catalyst and place it in the catalyst filling cylinder (5), and above the material-liquid distribution plate (6), cover the stainless steel sieve plate (8) above the catalyst, compress the catalyst with a compression spring, and then cover the reactor end cap on, tighten. The feed liquid containing 0.3mol / L hydroxylamine nitrate, 0.1mol / L hydrazine nitrate, 1.0mol / L nitric acid and 0.06g / L hexavalent uranium was preheated to 60°C, and passed through the feed liquid port (1 ) is pumped into the reactor to carry out the reaction, and the reacted solution flows out from the product outlet (9), and the content of hydrazine nitrate in the solution after the reaction is measured to be 0.0002 mol / L and the content of hydroxylamine nitrate to be 0.000005 mol / L.
Embodiment 2
[0093] Weigh 700g of catalyst and place it in the catalyst filling cylinder (5), and above the material-liquid distribution plate (6), cover the stainless steel sieve plate (8) above the catalyst, compress the catalyst with a compression spring, and then cover the reactor end cap on, tighten. The feed liquid containing 0.3mol / L hydroxylamine nitrate, 0.1mol / L hydrazine nitrate, 1.0mol / L nitric acid and 0.06g / L hexavalent uranium was preheated to 80°C, and passed through the feed liquid port (1 ) is pumped into the reactor to carry out the reaction, and the reacted solution flows out from the product outlet (9), and the content of hydrazine nitrate after the reaction is measured to be 0.00001 mol / L and the content of hydroxylamine nitrate to be 0.000008 mol / L.
Embodiment 3
[0095] Weigh 700g of catalyst and place it in the catalyst filling cylinder (5), and above the material-liquid distribution plate (6), cover the stainless steel sieve plate (8) above the catalyst, compress the catalyst with a compression spring, and then cover the reactor end cap on, tighten. The feed liquid containing 0.3mol / L hydroxylamine nitrate, 0.1mol / L hydrazine nitrate, 1.0mol / L nitric acid and 0.06g / L hexavalent uranium was preheated to 60°C, and passed through the feed liquid port (1 ) is pumped into the reactor to carry out the reaction, and the reacted solution flows out from the product outlet (9), and the content of hydrazine nitrate in the solution after the reaction is measured to be 0.0004 mol / L and the content of hydroxylamine nitrate to be 0.000009 mol / L.
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com