Medicine for promoting tissue cell regeneration
A technology of tissue regeneration and medicine, which is applied in the field of medicine, can solve the problem of few medicines, and achieve the effect of promoting healing and promoting activation and migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
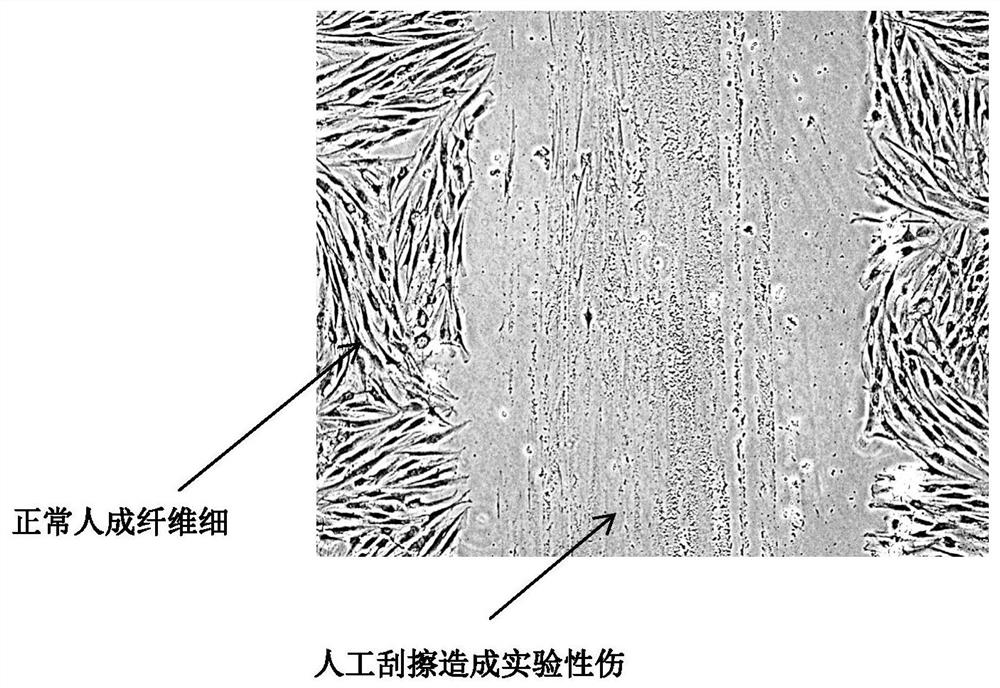
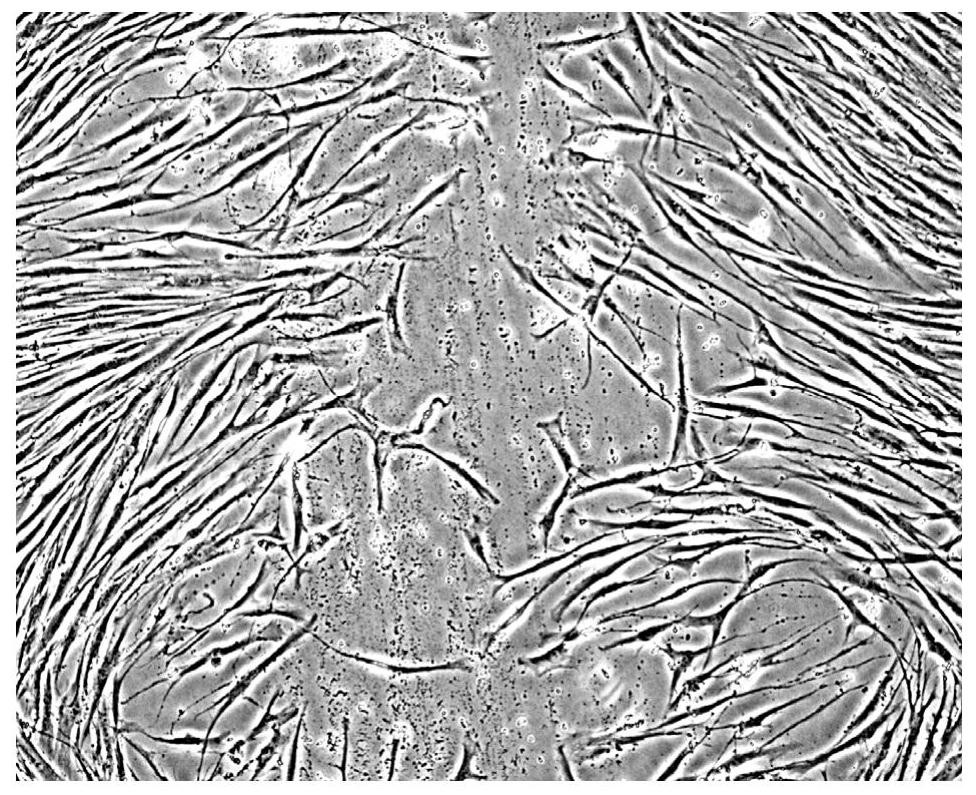
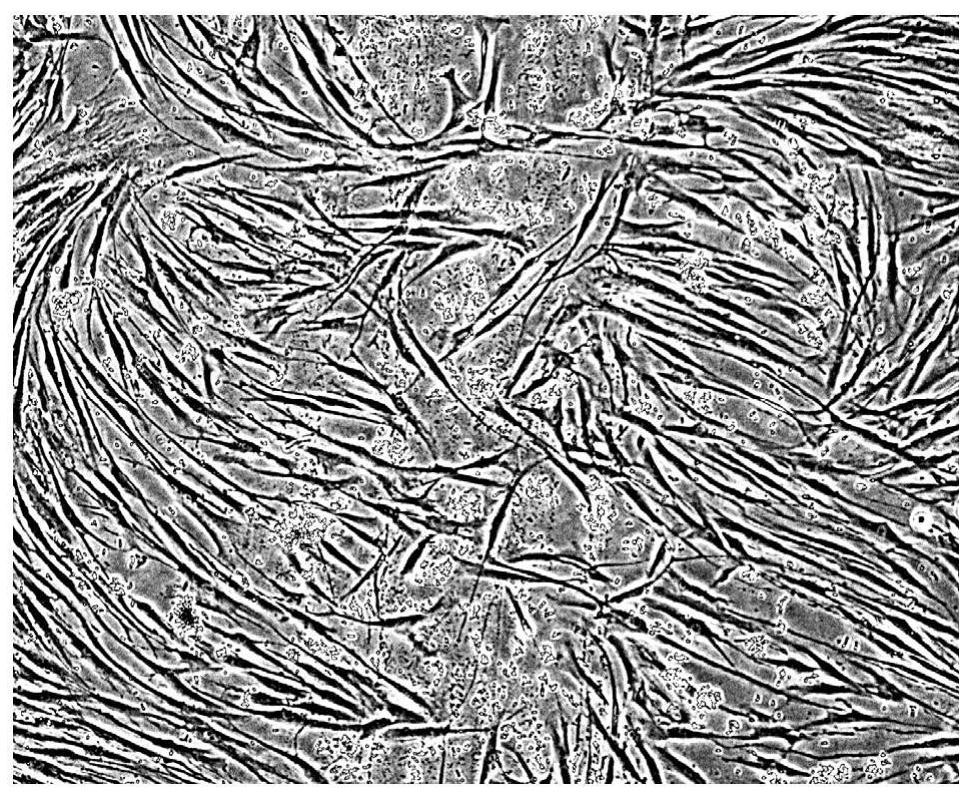
[0058] Human skin fibroblasts were suspended in DMEM medium with 10% bovine serum at a cell concentration of 2 × 10 5 / mL suspension, put it into a six-well plate with slides placed (the next step requires slide staining, etc., which cannot be operated in a six-well plate, put the suspension in a six-well plate with slides) cells were grown on slides in six-well plates) and incubated overnight in a 37°C incubator. When the cell conflux rate (confluncy) reaches 50%-70%, use a surgical sterilized blade to scratch hard in the glass slide to cause experimental wounds, such as figure 1 shown.
[0059] 2 mL of PBT with a concentration of 10 μg / mL was used to cover the glass slides scraped from the experimental wounds, sealed for water retention, and cultured in a 37°C incubator; at the same time, the control group was set as a control. The cells were cultured in a ℃ incubator, and the changes in the degree of confluence of fibroblasts in the experimental group and the control grou...
Embodiment 2
[0061] Healing effects of PBT on traumatic wounds in a zebrafish model:
[0062] Prepare 20 adult zebrafish with a weight difference of not more than 50g, and randomly divide them into two groups, each group of 10, named the first group and the second group respectively. The two groups of zebrafish were cultured in two culture tanks with a pH of 7.2 and a water temperature of 27 °C. The caudal fins of the first group of zebrafish were cut about 2-3 mm from the caudal end, and the second group of zebrafish were not treated with caudal fin cutting. The two groups of zebrafish were subdivided into two groups (the first group (1) and the first group (2), the second group (1) and the second group (2)), and placed in a petri dish with a diameter of 35 mm. The first (1) and second (1) groups were co-cultured with fluorescently labeled human neutrophils with PBT at a concentration of 10 mg / mL; the first (2) and second (2) only Cultured with human neutrophils without PBT. The change...
Embodiment 3
[0065] A total of 20 two-week-zero BALB / C mice, half male and half, weighing about 15-20 grams, were randomly divided into two groups (the drug experimental group and the control group, 10 mice each). Dissolve 200 μg of PBT in sterilized 20-30 μL of ethanol, prepare a drug solution, slowly push the drug solution into the tail vein of the mice, observe and record the changes in the behavior, body weight, and body hair of the mice. The body weight of the mice was recorded for 20 days, and it was found that within the effective dose of PBT, compared with the control group, the mice injected with the PBT drug had no significant change in body weight.
[0066] After 20 days, the mice were euthanized, and the brain, heart, gastrointestinal, lung, liver and other organs were removed, and the pathological changes were observed by staining. The pathological pictures of the brain staining of the mice in the drug experimental group and the control group are as follows: Figure 7 As show...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com