Adsorption particles for extracting lithium from natural brine based on molecular sieve adsorbent and preparation method of adsorption particles
A technology for adsorbing particles and molecular sieves, which is applied in the direction of adsorption of water/sewage treatment, chemical instruments and methods, and improvement of process efficiency. The effect of improving the strength of acid and alkali resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
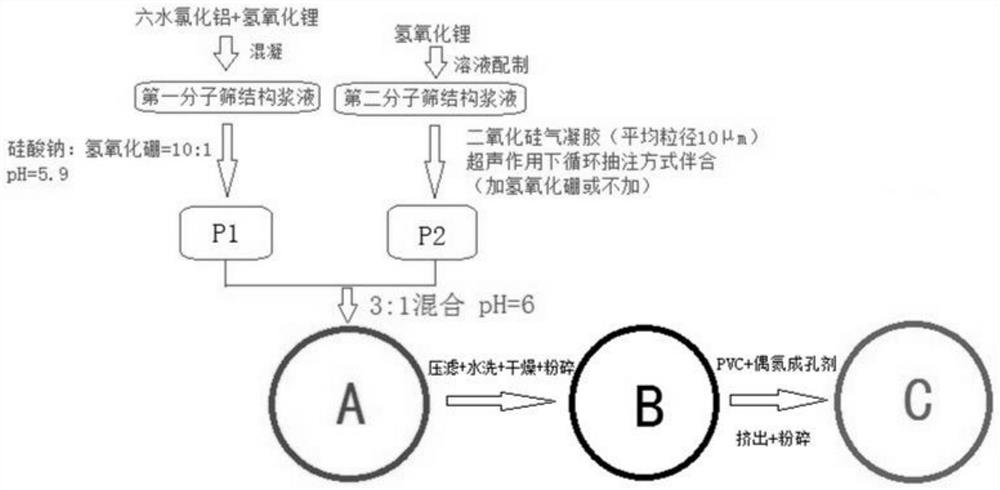
[0039] The present embodiment provides a preparation method flow of natural brine lithium extraction and adsorption particles based on molecular sieve adsorbent, such as figure 1 shown
[0040] First, aluminum chloride hexahydrate, lithium hydroxide and sodium silicate are respectively made into aqueous solutions; aluminum chloride hexahydrate solution is coagulated with lithium hydroxide solution to form a first molecular sieve structure slurry; lithium hydroxide is configured into a solution to form The second molecular sieve structure slurry.
[0041] Add sodium silicate: boron hydroxide mass ratio 10:1 solution to the first molecular sieve structure slurry and mix, and control the pH value to 5.9; add silica aerogel with an average particle size of 10 μm to the second molecular sieve structure slurry Particles (mass ratio is 8:1), and boron hydroxide (mass ratio with the slurry is 1:10) is added to mix, and then accompanied in a container with extraction under the action ...
Embodiment 2
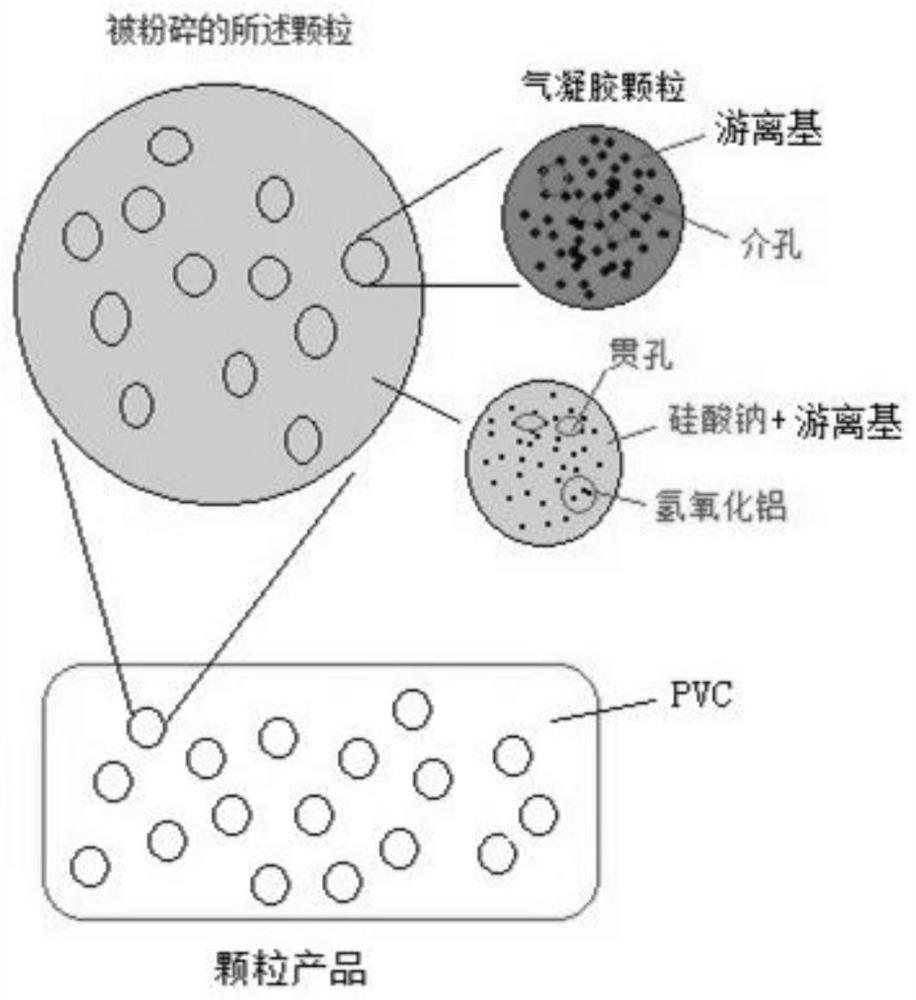
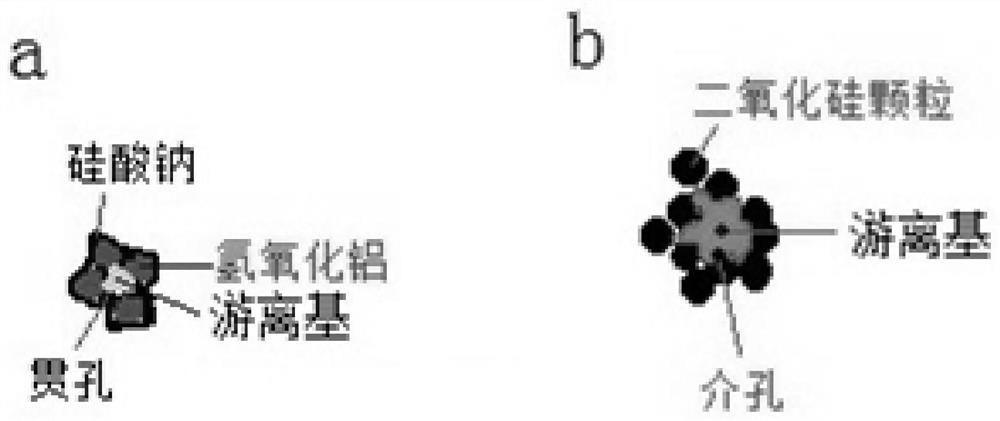
[0045] The only difference between this example and Example 1 is that boron hydroxide is not added to the second molecular sieve structure slurry. like figure 2 Shown are the schematic diagrams of the granular products of Examples 1 and 2, their microscopic particle structure and their composition, wherein the crushed particles comprise a sodium silicate-bonded aluminum hydroxide molecular sieve matrix and a silica aerosol. glue particles. The matrix is sodium silicate via such as image 3 (a) the through hole formed by the aluminum hydroxide particle skeleton bonded by sodium silicate and the hydrous lithium chloride free radical or hydrous lithium chloride-tetrahydroxyboron double free radical in the through hole formed by the schematic structure, Silica aerogel particles are image 3 (b) The three-dimensional network mesopores formed by the silica particles formed by the schematic structure and the hydrous lithium chloride free radicals or the hydrous lithium chloride...
Embodiment 3
[0047] The difference from Example 1 is that only the first molecular sieve structure slurry coagulated with aluminum chloride hexahydrate solution and lithium hydroxide solution is used to bond sodium silicate to form through-holes to fill aqueous lithium chloride free radicals. The lithium adsorption effect of Examples 1-3 is shown in Table 1
[0048] Table 1 Lithium ion adsorption rate and wear coefficient of Examples 1-3 compared with the results of the prior art
[0049]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com