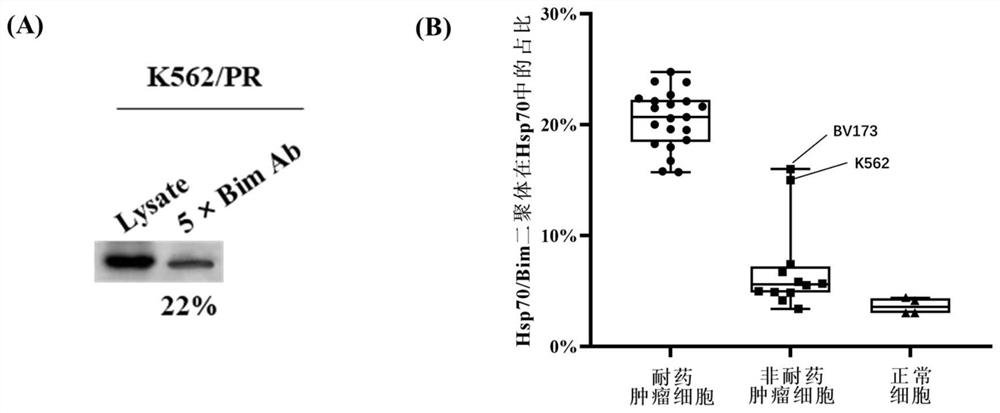
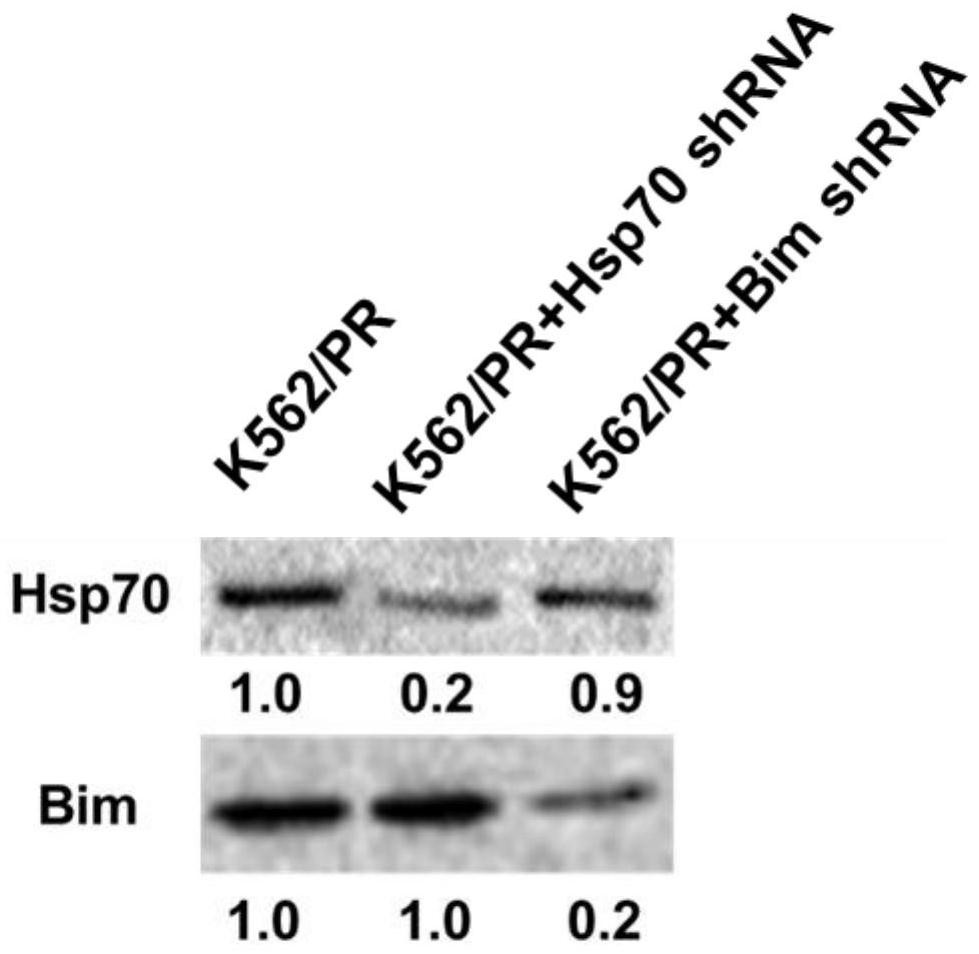
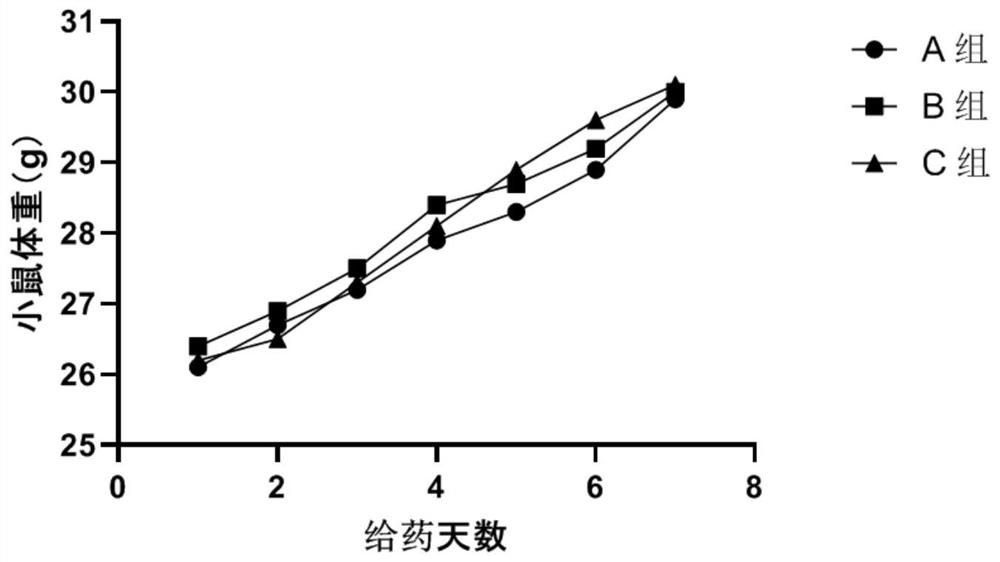
Application of 2-cyano phenalenone compound in preparation of drug for treating drug-resistant tumors
A kind of technology of cyanophenacene and compound, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Detection of the ability of the compounds of the present invention to dissociate Hsp70 / Bim dimers in vitro by fluorescence polarization analysis
[0033] A BimBH3 peptide with 21 amino acids (amino acids: 79-99: QEDIIRNIARHLAQVGDSMDR) was synthesized and labeled with 6-carboxyfluorescein succinimidyl ester (FAM) as a fluorescent tag (FAM-Bim) at the N-terminus. The reaction system used in the competition binding experiment was GST-Hsp70 protein (300 nM), and FAM-Bim polypeptide (20 nM) dissolved in reaction buffer (100 mM K 3 PO 4 , pH 7.5; 100 μg / mL bovine gamma albumin; 0.02% sodium azide). In a 96-well plate, 100 µL of the reaction system was added to each well, followed by 1 µL of stock solutions of the compounds to be tested (0-10 mM) dissolved in DMSO at various concentrations, with a final concentration of 0-100 µM. At the same time, two control groups were set up. One control group contained only Hsp70 and FAM-Bim in the reaction system (equivalent ...
Embodiment 2
[0037] Example 2: Construction of different drug-resistant cell lines
[0038] According to the mRNA expression data of about 1,000 tumor cell lines of 22 tumor types collected in the CCLE database, we mainly select tumor cell lines such as breast cancer, lung cancer, leukemia, and liver cancer to establish tumor cell lines that are resistant to various anticancer drugs.
[0039] The drug concentration gradient method was used to establish tumor cell lines resistant to targeted drugs and chemotherapeutic drugs with different mechanisms, including chronic myeloid leukemia (CML) resistant to the third-generation BCR-ABL kinase inhibitor Ponatinib. ) cell lines K562 / PR, BV173 / PR; cisplatin (DDP)-resistant CML cell lines K562 / DDP, BV173 / DDP; Bcl-2 targeting drug Venetoclax-resistant B-cell lymphoma (NHL) cell lines and Acute lymphoma leukemia (ALL) cell lines Ri-1 / VR, RS4; 11 / VR; etoposide-resistant NHL and ALL cell lines Ri-1 / ER, RS4; 11 / ER; tamoxib Tamoxifen-resistant breast ca...
Embodiment 3
[0041] Example 3: Detection of apoptosis of tumor drug-resistant cell lines induced by the compounds of the present invention by flow cytometry
[0042] Apoptosis is one of the basic characteristics of cells, and it plays a very important role in embryonic development, tissue repair, and stability of the internal environment. In normal cells, phosphatidylserine (PS) is only distributed in the inner side of the lipid bilayer of the cell membrane, while in the early stage of apoptosis, the phosphatidylserine (PS) in the cell membrane is turned from the inside to the outside of the lipid membrane. Annexin V is a Ca-dependent phospholipid-binding protein with a molecular weight of 35-36 kD, which can specifically bind to PS that is flipped to the outside of the membrane during apoptosis with high affinity. Using FITC-labeled annexin V as a fluorescent probe, the occurrence of apoptosis can be detected by flow cytometry or fluorescence microscopy. PI (propidium iodide) is a DNA dy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com