Near-infrared molecular probe capable of reversibly responding to ClO-/GSH oxidation reduction as well as preparation method and application of near-infrared molecular probe
A molecular probe and near-infrared technology, applied in the field of near-infrared molecular probes and their preparation, can solve the problems that it is difficult to reveal the oxidation-reduction state, and the probe cannot monitor oxides and reduced substances synchronously, achieving good results and easy The effect of promotion and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Preparation of Example 1 Compound 1:
[0062] Dissolve 3,5-di-tert-butyl-4-hydroxybenzaldehyde (0.02mol) and 4-methoxyacetophenone (0.02mol) in 20 mL of ethanol and add 3 g of potassium hydroxide (0.05mol), the resulting mixture After stirring at room temperature for 1 h, the precipitated product was filtered, washed with 20 mL of ethanol and dried under reduced pressure to obtain compound 1 (565 mg, yield 33%) as a pale yellow solid.
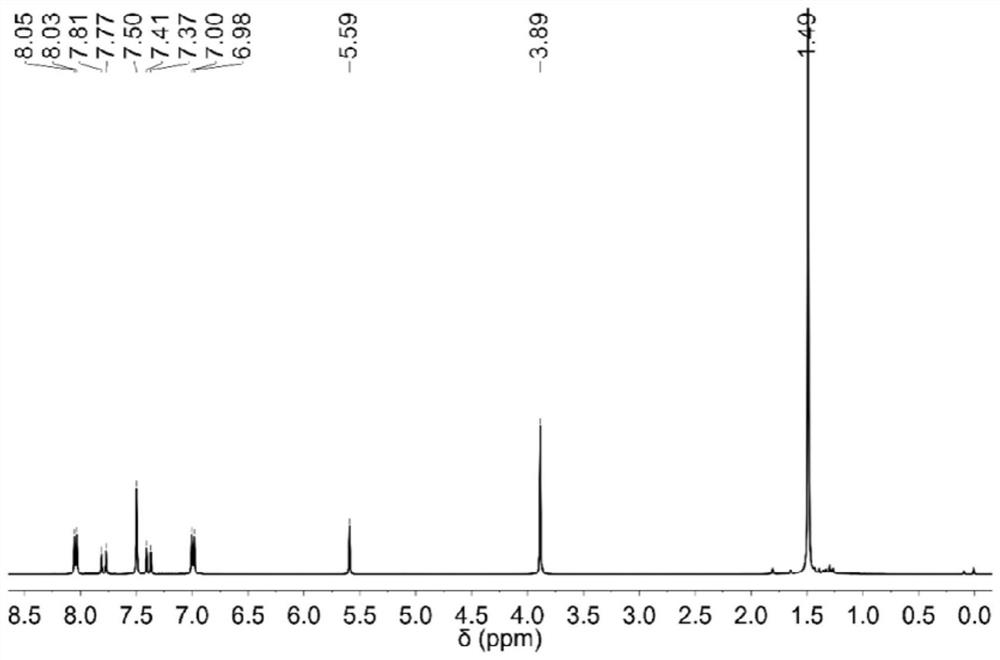
[0063] The hydrogen nuclear magnetic spectrum and carbon spectrum of compound 1 prepared in Example 1 are as follows figure 1 , 2 shown, figure 1 for the hydrogen spectrum results. 1 H NMR (400MHz, CDCl 3 , 25℃, TMS), δ=8.07-8.01(m, 2H), 7.79(d, J=15.6Hz, 1H), 7.58-7.51(m, 2H), 7.49(s, 1H), 7.23(d, J=8.0Hz, 2H), 7.01-6.96 (m, 2H), 3.89 (s, 3H), 2.39 (s, 3H) ppm; figure 2 For the carbon spectrum results, 13 C NMR (101 MHz, CDCl 3 , 25°C, TMS), δ=188.8, 163.4, 144.1, 140.8, 132.3, 131.2, 130.8, 129.7, 128.4, 120.9, 113.8, 55.5, 21...
Embodiment 2
[0064] Preparation of Example 2 Compound 2:
[0065] Compound 1 (20 mmol), nitromethane (10 mL) and diethylamine (10 mL) were dissolved in methanol (30 mL) and heated to reflux for 10 h. After cooling at room temperature, the solvent was removed in vacuo and washed with water. The organic layer was dried over anhydrous sodium sulfate and concentrated to give compound 2 (565 mg, yield 33%) as a yellow oil.
[0066] Compound 2 prepared in Example 2 has a hydrogen nuclear magnetic spectrum and a carbon spectrum such as image 3 , 4 shown, image 3 for the hydrogen spectrum results. 1 H NMR (400MHz, CDCl 3 , 25℃, TMS), δ=7.94-7.87(m, 2H), 7.15(q, J=8.2Hz, 4H), 6.94-6.89(m, 2H), 5.29(s, 1H), 4.82(dd, J=12.4, 6.5Hz, 1H), 4.65 (dd, J=12.4, 8.2Hz, 1H), 4.22-4.13 (m, 1H), 3.86 (s, 3H), 3.40-3.33 (m, 1H), 2.31 (s, 3H) ppm; Figure 4 For the carbon spectrum results, 13 C NMR (101 MHz, CDCl 3 , 25°C, TMS), δ=195.5, 163.8, 137.4, 136.4, 130.4, 129.6, 127.0, 113.9, 79.8, 55.5, 53....
Embodiment 3
[0067] Example 3 Synthesis of near-infrared molecular probe AzaBDP-DiOH:
[0068] Compound 2 (1.0 mmol) and ammonium acetate (20 mmol) were dissolved in ethanol (20 mL) at reflux for 9 h, cooled to room temperature and concentrated to about 5 mL. The precipitate was washed with ethanol and filtered to obtain the blue-black solid product azadipyrrolene, which was used directly without purification. To toluene (100 mL) was added the obtained azabipyrrolidine (0.23 mmol, 100 mL), and triethylamine (4 mL) and boron trifluoride diethyl ether (6 mL) were added. The mixture was stirred at 60 °C for 2 h. After cooling to room temperature, the solvent was removed under vacuum. The residue was washed with ethanol and filtered to obtain a red solid, which is the near-infrared photoacoustic probe AzaBDP-DiOH (83% yield).
[0069] The hydrogen nuclear magnetic spectrum, carbon nuclear magnetic spectrum and mass spectrum of the probe prepared in Example 3 are as follows: Figure 5 , 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com