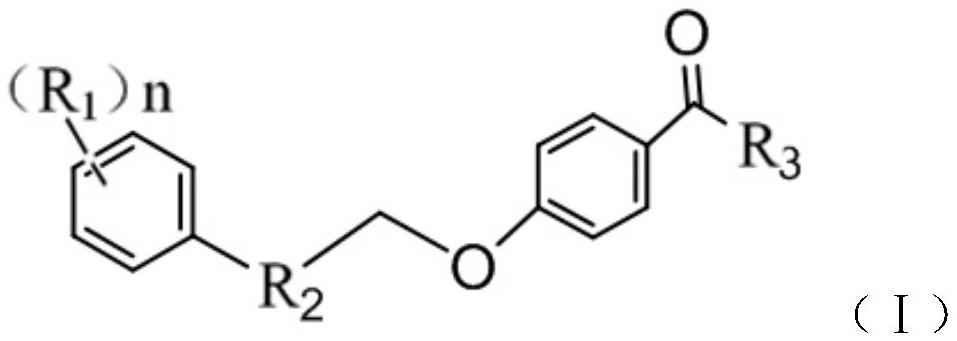
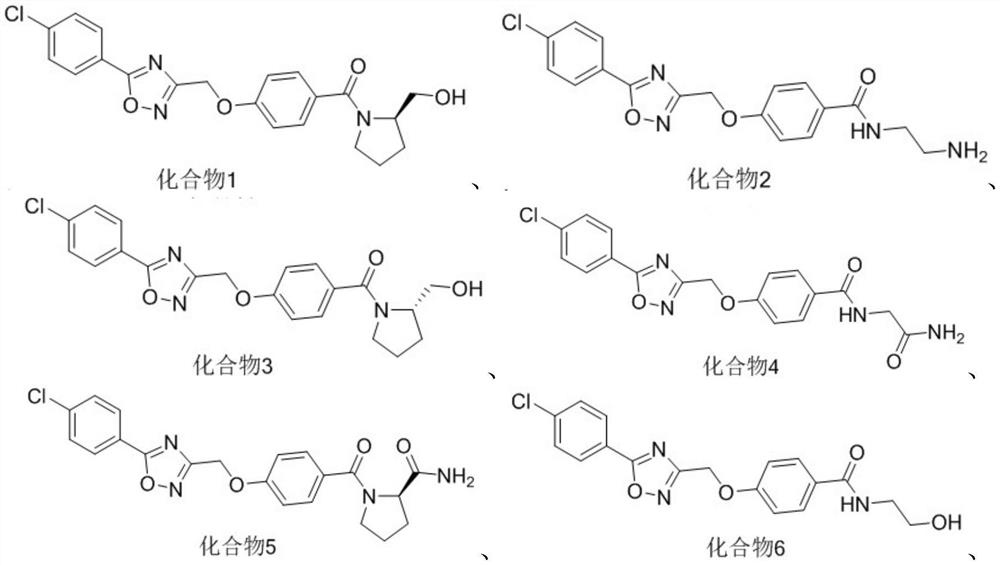
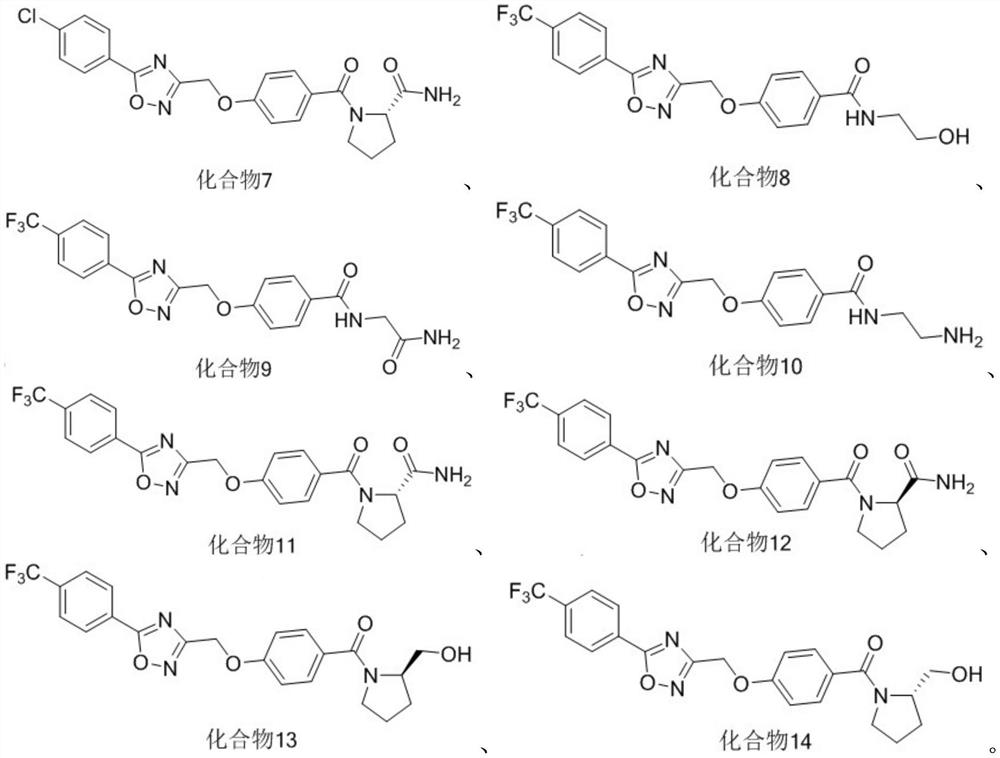
Five-membered heterocycle substituted benzamide compound as well as preparation method and application thereof
A compound and single substitution technology, applied in the field of benzamide compounds and their preparation, can solve the problems of low specificity and low potency of SphK2 inhibitors, and achieve the effect of strong inhibitory effect and multiple selection possibilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of series compound shown in formula (I)
[0040] S1), 3g (20mmol) methyl p-hydroxybenzoate, 5.4g (40mmol) potassium carbonate, 0.4g (2mmol) potassium iodide were added to dissolve in a 15mL eggplant-shaped bottle filled with DMF, and then 1.6g (24mmol) was added Chloroacetonitrile was heated to reflux at 85°C for 4 hours, and the end point of the reaction was detected by TLC. The solvent was removed under reduced pressure to obtain a crude product, which was recrystallized from ethyl acetate to obtain 3.2 g of product C with a yield of 85%. 1 H NMR (600MHz, CDCl 3 )δ8.07–8.04(m,2H), 7.02–7.00(m,2H), 4.83(s,2H), 3.91(s,3H).
[0041] S2), dissolve 2.86g (15mmol) of compound C with 20mL of absolute ethanol solution, add 5.2g (75mmol) of hydroxylamine hydrochloride and 8.8g (105mmol) of sodium bicarbonate, heat and reflux at 85°C for 1 hour, and then detect the reaction by TLC At the end point, filter while hot, wash the filter cake 3-4 times...
Embodiment 2
[0065] Embodiment 2: The activity research of series compound of the present invention to SphK2
[0066] (1) Experimental materials: target compound and positive control PF543 (Target mol, T8840), sphingosine kinase 2 kit (Cayman, 701870), microplate reader (Bio-Stack) and black 96-well plate.
[0067] (2) Experimental method:
[0068] Compound 3, which has strong inhibitory effects on the three cancer cells, was selected, and the inhibition rates at different concentrations of 5 μM, 10 μM, 25 μM, 50 μM, 80 μM, and 100 μM were respectively determined. The experimental results are shown in Table 2.
[0069] Table 2
[0070] concentration 5μM 10μM 25μM 50μM 80μM 100μM Inhibition rate(%) 66.7 66.7 88.9 >100 >100 >100
[0071] All samples were tested for single well inhibition rate at a concentration of 10 μM, and the experimental results are shown in Table 3.
[0072] table 3
[0073] Numbering Inhibition rate(%) 6 60 8...
Embodiment 3
[0079] Example 3: Research on the effect of the series of compounds of the present invention on the proliferation of cancer cells
[0080] (1) Experimental materials: target compound and positive control PF543, trypsin, cleaning solution PBS, fetal bovine serum (Gemini), human histiocytic lymphoma cell U937, human breast cancer cell MCF-7 and human gastric cancer cell MGC-308 ( Shanghai Chinese Academy of Sciences Cell Bank), microplate reader (thermo scientific) and 96-well plate, DMEM medium and 1640 medium (Solarbio, where the final concentrations of penicillin and streptomycin are 100U / mL and 100μ / mL, respectively).
[0081] (2) Experimental method:
[0082] The cells grown to the logarithmic phase were made into uniformly dispersed single-cell suspension, and 90 μL of single-cell suspension per well was about (2-5)×10 4 cells / ml were seeded into 96-well plates in 5% CO 2 , Incubate in a 37°C incubator for 48h. After the cells adhered to the wall, add 10 μL of samples o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com