Organic luminescent material with high exciton utilization rate and preparation method and application thereof
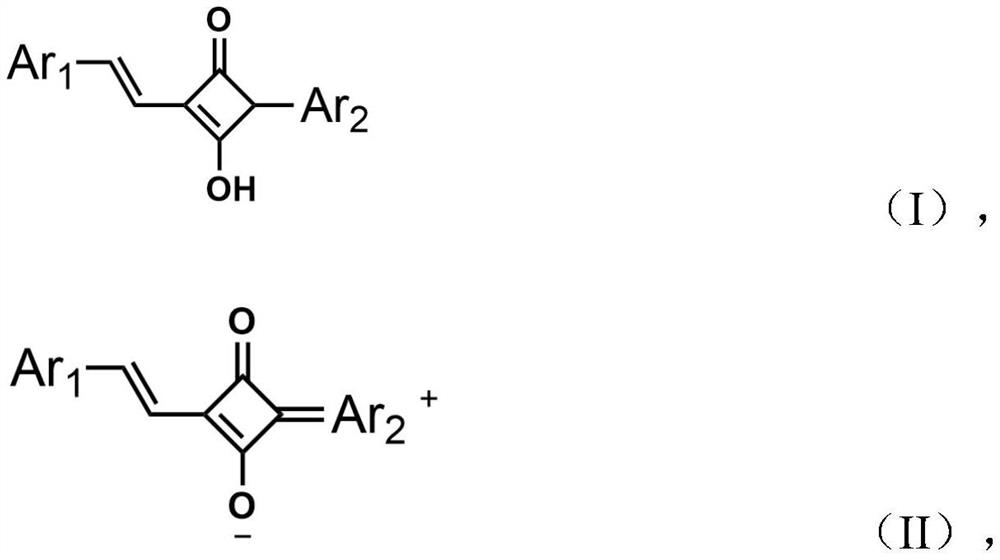
A technology of luminescent materials and utilization rate, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of few red thermal exciton fluorescent materials, the performance needs to be improved, the number of materials is scarce, etc., to achieve a single molecular structure. Clear, commercial application, excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
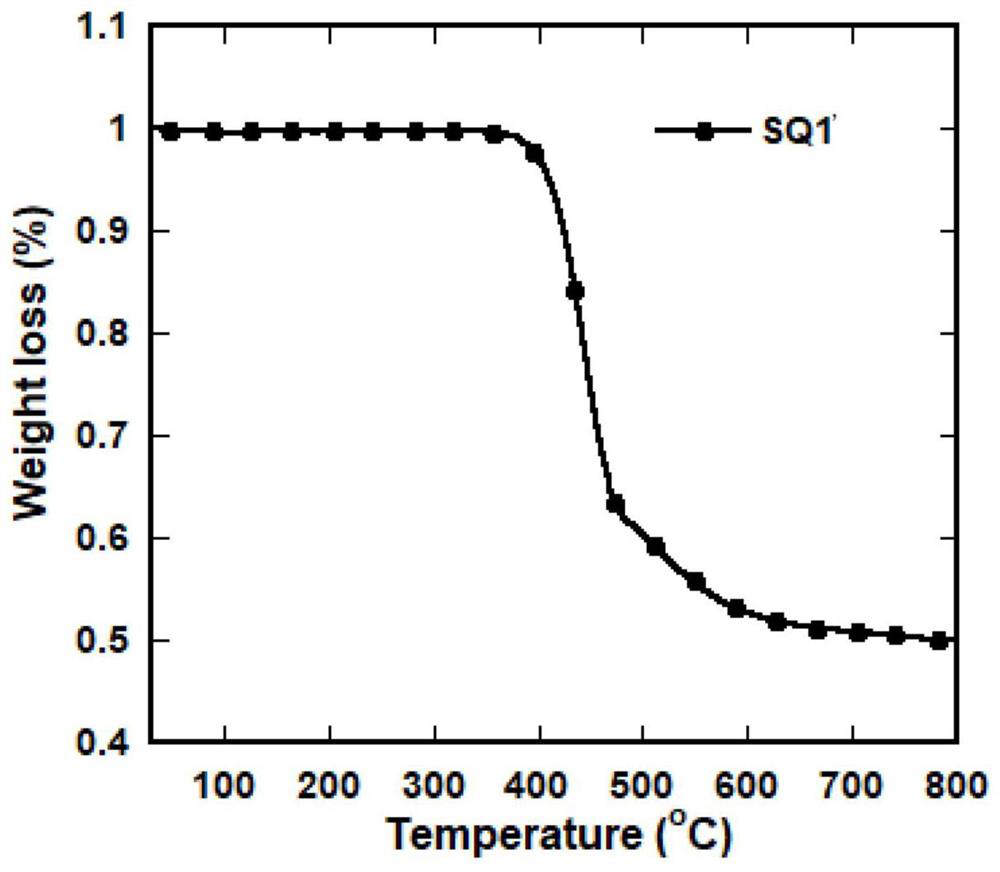
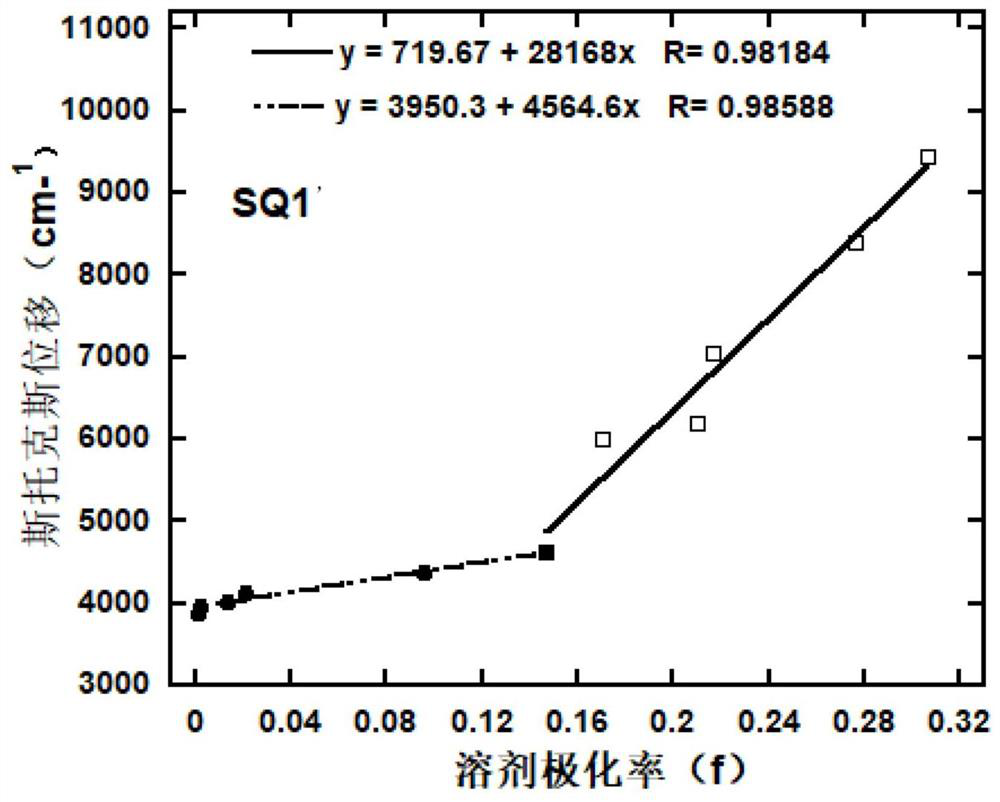
[0084] Embodiment 1: Preparation of red thermal exciton fluorescent material SQ1'
[0085] (1) Preparation of compound M3'
[0086] Under argon atmosphere, 9,10-phenanthrenequinone (4.16g, 20mmol), 4-tert-butylaniline (14.9g, 100mmol), p-bromobenzaldehyde (3.68g, 20mmol), ammonium acetate (6.16g, 80mmol ) was dissolved in 150mL acetic acid, and reacted at 120°C for 12 hours. After stopping the reaction, quench the reaction with water, spin to dry the solvent, extract with dichloromethane and dry with anhydrous magnesium sulfate. After the solution is concentrated, a khaki liquid is obtained, which is purified by silica gel column chromatography, and a mixed solvent of petroleum ether and dichloromethane is used. (Volume ratio 4:1) was used as eluent to obtain a white solid with a yield of 82%. 1 HNMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product M3', and the chemical reaction equation of the preparation process is as follo...
Embodiment 2~5
[0122] Examples 2-5: Preparation of organic luminescent materials SQ2'-SQ5' with high exciton utilization
[0123] Similarly, on the basis of Example 1, the raw materials in step (5) are replaced, and other conditions are not changed, so that different red thermal exciton fluorescent materials can be obtained, the raw materials before replacement, the raw materials after replacement , the product obtained after replacement and the productive rate are shown in Table 6 below:
[0124] In the step (3) of the embodiment 1 of table 6, the obtained product structure and the productive rate after the replacement of raw materials
[0125]
[0126]
Embodiment 6~9
[0127] The preparation of embodiment 6~9 compound SQ6'~SQ9'
[0128] Under argon atmosphere, compound M10'(1.38g, 2.5mmol) and compound M11'(0.69g, 2.5mmol) were dissolved in 10mL n-butanol and 10mL toluene solution, reacted at 100°C for 24h, after stopping the reaction, quenched with water The reaction was quenched, extracted with dichloromethane and dried with anhydrous magnesium sulfate. After the solution was concentrated, a khaki liquid was obtained, which was purified by silica gel column chromatography, and a mixed solvent of petroleum ether and dichloromethane (volume ratio 2:1) was used for eluting agent, a red solid SQ6' was obtained with a yield of 45%. 1 H NMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product SQ6', and the chemical reaction equation of the preparation process is as follows:
[0129]
[0130] Similarly, on the basis of Example 6, by replacing the raw materials in it, and without changing other con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com