Application and method of E199L protein in promoting apoptosis
A cell and apoptosis technology, applied in the field of cell biology, can solve problems such as decreased activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Materials and methods
[0054] 1.1 Construction of recombinant plasmids
[0055] 94 expression ASFV encoded proteins (pCAGGS-Flag-X) and BCL-2 family members (pCAGGS-HA / Flag-Y, BAK, BAX, BCL-XL, MCL-1, BCL-W, BCL-2, BCL-2A1) plasmids. E199L and its mutant plasmids (pEGFP-E199L, pEGFP-E199L-dMTD, pEGFP-E199L-dBH3, pEGFP-E199L-dBH1, pCAGGS-HA-E199L) were constructed into pEGFP-C1 or pCAGGS- HA carrier. All constructed plasmids were verified by DNA sequencing.
[0056] 1.2 cells
[0057] HEK293T and HeLa cells were stored in our laboratory at 37°C, 5% CO 2 cultured in an incubator with 10% fetal bovine serum, 100 U / mL penicillin and 100 mg / mL streptomycin in the medium. HeLa-ΔBAK and its parental cells were purchased from EDIGENE (catalogue number: LS0032850802A).
[0058] 1.3 Antibody
[0059] The primary antibodies used in this study were specific TOM20 (612278, BD Biosciences), PDI (MA3-19, Thermo), HA (3724S, Cell Signaling Technologies), FLAG (14793...
Embodiment 2
[0078] Example 2: ASFV E199L induces cell death in vitro
[0079] according to figure 1 Based on the results, we proposed that the protein encoded by ASFV may be involved in the process of ASFV-induced cell death. In order to verify this hypothesis, 94 plasmids expressing ASFV-encoded proteins were transfected into HEK293T cells, and then used CellTiter-Glo TM Fluorescent cell viability assay. Screening results showed that the overexpression of 4 genes (B117L, CP123L, E183L, E199L) had a significant effect on cell viability ( figure 1 A). In particular, E199L-induced cell death was as high as 60%. Therefore, this study chose E199L for further study. Our results showed that in PAM cells infected with the HLJ / 18 isolate, the mRNA level of E199L was significantly increased starting from 12 hours post-infection (HPI) ( figure 1 B), which indicates that E199L is a late gene expressed by the virus. To further verify the effect of E199L on inducing cell death, the E199L exp...
Embodiment 3
[0080] Example 3: Cell death induced by ASFV E199L presents apoptosis characteristics
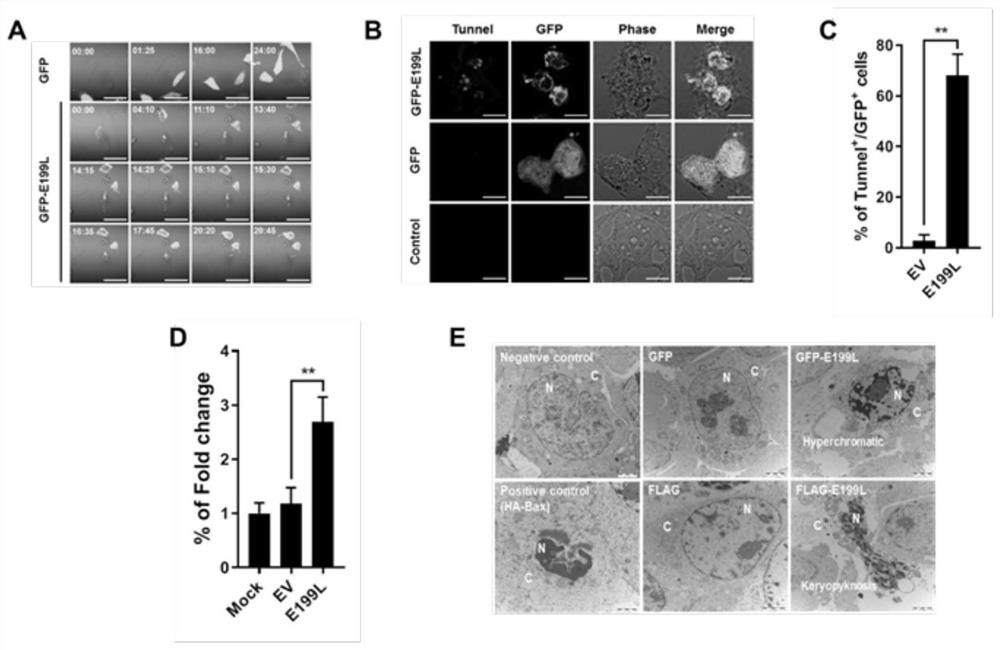
[0081] To investigate whether E199L induces cell death by inducing apoptosis in vitro, time-lapse confocal microscopy, TUNEL labeling and Annexin V-FITC / PI staining were used to detect the morphological changes of apoptosis, DNA fragments, phosphatidylserine (PS ) eversion. The characteristic morphological changes of apoptosis were observed by real-time confocal microscopy. After E199L aggregated in HeLa cells, the cell morphology began to shrink, bleb, and eventually form apoptotic bodies ( figure 2 A). In addition, apoptosis-induced DNA fragmentation was marked by TUNEL assay. HEK293T cells overexpressing E199L produced TUNEL positive signal ( figure 2 B), the proportion of TUNEL positive cells is as high as 60%, which is significantly higher than that of HEK293T cells expressing GFP ( figure 2 C). In addition, we also found that the apoptosis level of cells transfected with E199...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com