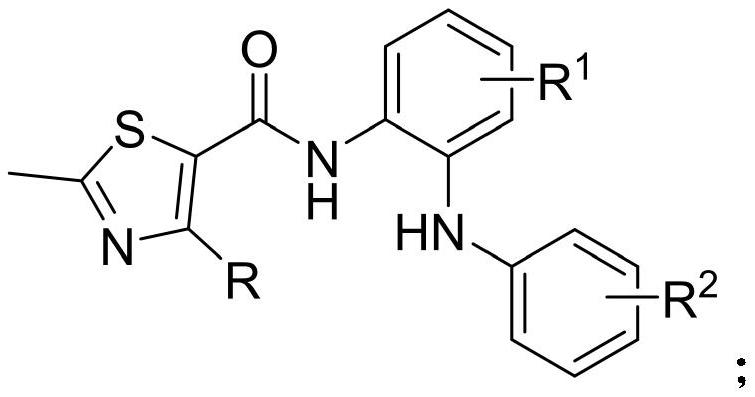
Diphenylamino-containing thiazole amide compound as well as preparation method and application thereof
A technology based on thiazole amide and diphenylamine, which is applied in the field of thiazole amide compounds and their preparation, achieving good results, simple preparation methods, and novel structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] N 1 Preparation of -(4-chlorophenyl)-1,2-phenylenediamine (A)
[0028]
[0029] (1)N 1 Preparation of -(4-chlorophenyl)-2-nitroaniline
[0030] Take 2-chloronitrobenzene (10.0mmol) and 4-chloroaniline (15.0mmol) in a single-necked bottle, add potassium carbonate and polyethylene glycol-1000, after the addition is complete, heat up to 185°C, react for 20h, and detect by TLC. After completion of the reaction, cool to room temperature, add ethyl acetate to extract 3 times, combine the organic layers, and wash with saturated brine, dry over anhydrous sodium sulfate, suction filter, precipitate to obtain an oil, and purify by column chromatography to obtain N 1 -(4-Chlorophenyl)-2-nitroaniline, yield 58.5%.
[0031] (2)N 1 Preparation of -(4-chlorophenyl)-1,2-phenylenediamine (A)
[0032] N 1 -(4-Chlorophenyl)-2-nitroaniline (5.0mmol) was dissolved in 75% ethanol (20mL), then ammonium chloride (15.0mmol) and reduced iron powder (15.0mmol) were added, after addition,...
Embodiment 2
[0034] N 1 Preparation of -(4-chlorophenyl)-4-fluorobenzene-1,2-diamine (B):
[0035]
[0036] (1) Preparation of N-(4-chlorophenyl)-5-fluoro-2-nitroaniline
[0037] 2,4-Difluoronitrobenzene (10.0mmol), substituted aniline (15.0mmol) and potassium fluoride (10.0mmol) were stirred in a 100mL round bottom flask at 160°C for 14 hours without solvent. Cool and quench the reaction with water, add ethyl acetate to extract 3 times, combine the organic layers, dry over anhydrous sodium sulfate, filter out anhydrous sodium sulfate, precipitate and dissolve with ethanol, decolorize with activated carbon, remove the activated carbon by suction and recrystallize or use Pure petroleum ether column chromatography gave N-(4-chlorophenyl)-5-fluoro-2-nitroaniline in a yield of 80.6%.
[0038] (2)N 1 Preparation of -(4-chlorophenyl)-4-fluorobenzene-1,2-diamine (B)
[0039] Dissolve N-(4-chlorophenyl)-5-fluoro-2-nitroaniline (5.0mmol) in 75% ethanol (20mL), then add ammonium chloride (15....
Embodiment 3
[0041] Preparation of thiazole amide compound (I-4) containing diphenylamino group
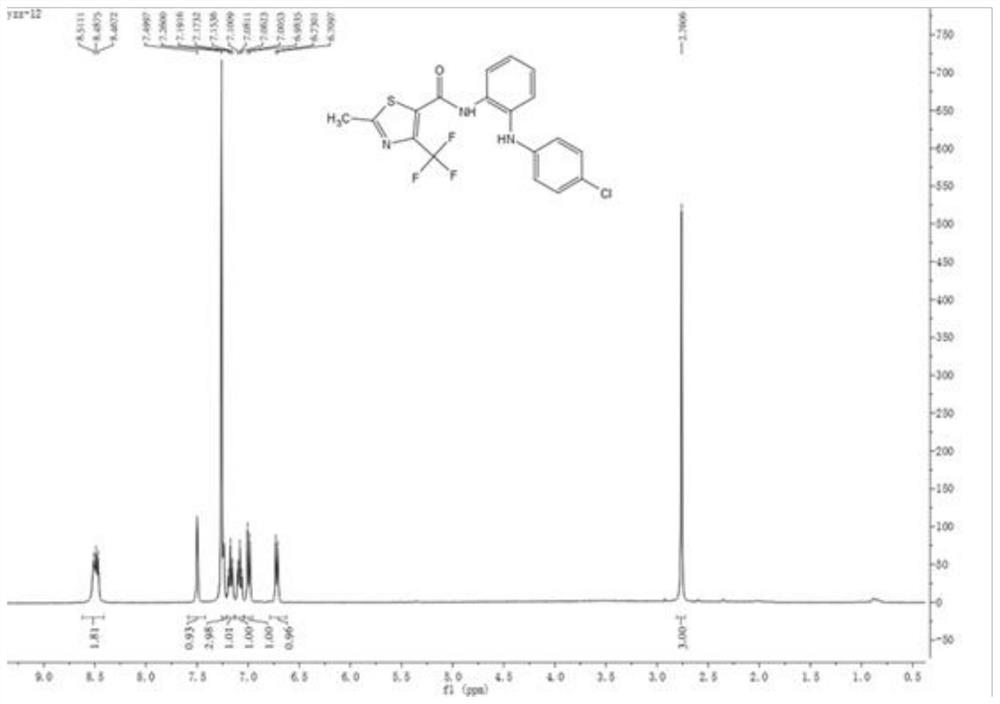
[0042] Add N to the one-necked bottle 1 -(4-Chlorophenyl)-1,2-phenylenediamine (A) (1.0mmol), add 20mL of acetonitrile to dissolve, slowly add 2-methyl-4-trifluoromethylthiazole-5-methanol dropwise at room temperature Chlorine (1.2mmol), after dropping, the temperature was raised to reflux for 3h, monitored by TLC, the reaction was completed, acetonitrile was recovered by precipitation, extracted 3 times with ethyl acetate, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and suction filtered. The crude product was obtained by precipitation, and the thiazole amide compound (I-4) containing diphenylamino group was obtained by column chromatography. The product characterization data was: white solid, yield 73.2%, m.p.146.5-147.0°C; 1 H NMR (400MHz, CDCl 3 )δ8.59-8.43(m,2H,C 6 h 4 ),7.50(s,1H,NH),7.24(s,3H,CONH+C 6 h 4 ),7.17(t,J=7.6Hz,1H,C 6 h 4 ),7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com