Theophylline acetic acid derivative with antitumor activity and preparation method thereof
A technology of theophylline acetic acid and its derivatives, which is applied in the fields of antineoplastic drugs, organic chemistry, drug combinations, etc., can solve the problems of large adverse reactions and easy drug resistance, and achieve novel molecular structure, low toxicity of normal cells, and good The effect of inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] At room temperature, theophyllineacetic acid (compound 1 (5 g, 0.02 mol)), 3-aminophenylacetylene (3.69 g, 0.03 mol), HATU (12.96 g, 0.03 mol) and DIPEA (8.13 g, 0.06 mol) and Solvent DMF250mL was added to a 500mL reaction flask together, and stirred for 24 hours under nitrogen protection. Using thin layer chromatography (TLC) monitoring, after 24 hours, the reaction was complete, and the reaction solution was light brown. Concentrate under reduced pressure to remove DMF, add dichloromethane to extract the reaction solution (150 mL×3), combine all organic phases, wash with saturated sodium chloride (150 mL×2) to pH=7, and concentrate to obtain a very viscous brown-yellow product. Under ultrasonic vibration, methanol was slowly added dropwise, and a solid was precipitated. Then stand, suction filtration, and drying to obtain the condensation intermediate of theophylline acetic acid and 3-aminophenylacetylene (compound 2, 3.64 g). LC-MS m / z (%): 337 [M+H] +...
Embodiment 2
[0033]
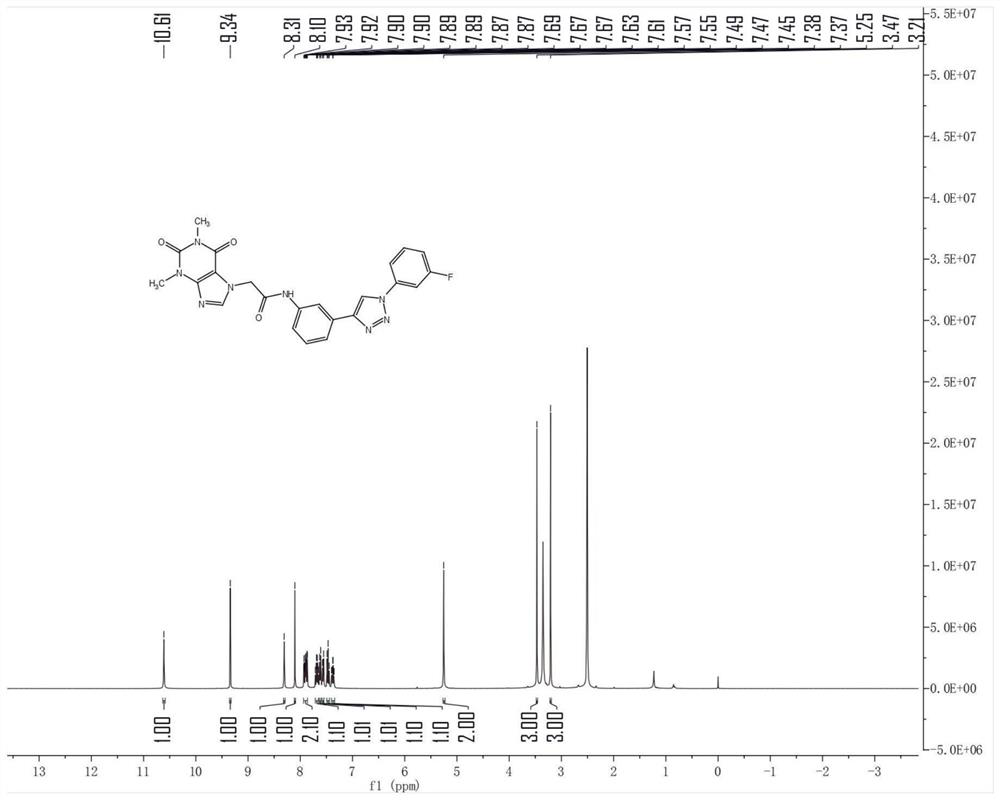
[0034] In the reaction flask, add compound 2 (1 g), 1 g of 3-chloro-4-fluorobenzyl azide, 70 mL of tert-butanol, 70 mL of water, 70 mL of tetrahydrofuran, 0.5 g of anhydrous copper sulfate and 1 g of sodium ascorbate in sequence. The reaction was carried out under the condition of ℃ for 6 hours, the raw materials were completely reacted, 100 mL of dichloromethane was added, and the reaction solution was filtered to obtain a yellow liquid. The organic phase was separated, and the aqueous phase was extracted twice with 50 mL of dichloromethane. The combined organic phases were dried over anhydrous magnesium sulfate. , concentrated under reduced pressure to obtain a solid, which was separated by chromatography to obtain compound J1 (1.03 g). 1 H NMR (400MHz, DMSO-d 6 )δ10.55(s, 1H), 8.64(s, 1H), 8.18(s, 1H), 8.09(s, 1H), 7.67(d, J=9.0, 1H), 7.52(d, J=7.5, 2H), 7.47–7.37(m, 3H), 5.65(s, 2H), 5.23(s, 2H), 3.46(s, 3H), 3.20(s, 3H).
Embodiment 3
[0036]
[0037] In the reaction flask, add compound 2 (1 g), 1 g of 2-trifluoromethylbenzyl azide, 70 mL of tert-butanol, 70 mL of water, 70 mL of tetrahydrofuran, 0.5 g of anhydrous copper sulfate and 1 g of sodium ascorbate in sequence. The reaction was carried out under the conditions for 6 h, the raw materials were completely reacted, 100 mL of dichloromethane was added, the reaction solution was filtered to obtain a yellow liquid, the organic phase was separated, the aqueous phase was extracted twice with 50 mL of dichloromethane, and the combined organic phases were dried over anhydrous magnesium sulfate, Concentration under reduced pressure gave a solid, which was separated by column chromatography to give compound J2 (0.96 g). 1H NMR (400MHz, DMSO-d6)δ10.55(s,1H), 8.61(s,1H), 8.18(s,1H), 8.09(s,1H), 7.83(d,J=7.7,1H), 7.70 (d, J=15.1, 1H), 7.57 (d, J=32.7, 3H), 7.40 (d, J=15.8, 1H), 7.24 (d, J=7.7, 1H), 5.84 (s, 2H) , 5.23(s, 2H), 3.46(s, 3H), 3.20(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com