Preparation method, product and application of a class of thiophene-linked 1,3,4-oxadiazole carboxamides
A technology of oxadiazole carboxamide and thiophene carboxyl hydrazide is applied in the field of pesticide synthesis and achieves the effects of novel molecular structure, easy control of reaction conditions and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
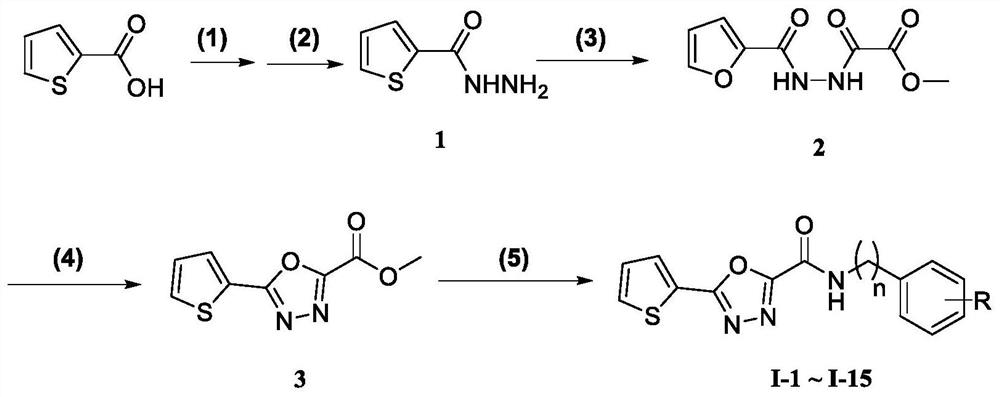
[0043] The schematic diagram of the preparation method of the thiophene-linked 1,3,4-oxadiazole carboxamide compounds of the present invention is shown in figure 1 , including the following steps:
[0044] (1) 2-thiophenecarboxylic acid is esterified to synthesize 2-thiophenecarboxylic acid ester;
[0045] (2) 2-thiophenecarboxylate undergoes hydrazinolysis reaction to obtain 2-thiophenecarboxyhydrazide (1);
[0046] (3) 2-thiophenecarboxyl hydrazide reacts with oxalyl chloride monomethyl ester to generate bisamide intermediate (2);
[0047] (4) The bisamide intermediate (2) is reacted with phosphorus oxychloride to obtain thiophene-1,3,4-oxadiazole formate (3);
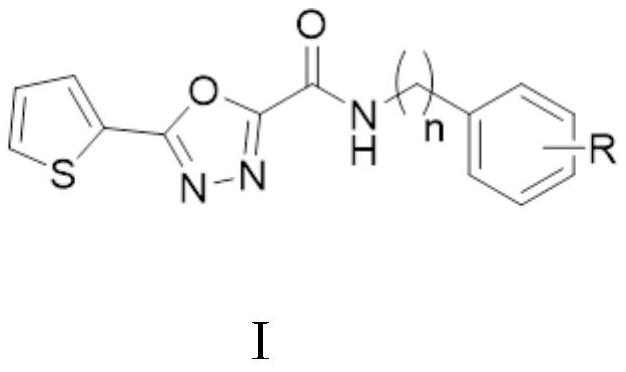
[0048] (5) Thiophene-1,3,4-oxadiazole carboxamide was reacted with substituted benzylamine to synthesize thiophene-1,3,4-oxadiazole carboxamide.
Embodiment 1
[0050] Preparation of 1,3,4-oxadiazole methyl ester of thiophene
[0051] Take 2-thiophenecarboxylic acid (6.41g, 50mmol) in a 250mL single-neck flask, add 100mL methanol and catalytic amount of concentrated sulfuric acid (0.5g, 5mmol), add, heat up to reflux temperature, react for 6h, TLC detection, the reaction is completed, cooled After reaching room temperature, saturated sodium bicarbonate solution was added dropwise with stirring until pH=7, ethyl acetate was added for extraction (50 mL×3), the organic layers were combined, washed with saturated brine (100 mL×2), dried over anhydrous sodium sulfate, and extracted. Filtration and desolubilization gave 6 g of oil.
[0052]Dissolve 6 g of the oily substance in 60 mL of ethanol, then add 85% hydrazine hydrate (7.94 g, 211 mmol), and after the addition, reflux for 5 h, TLC detects that the reaction of the raw materials is complete, cool, and rotate to concentrate to remove most of the ethanol. , washed with water (10 mL×3), ...
Embodiment 2
[0056] Preparation of Thiophene-1,3,4-oxadiazolecarboxamide
[0057]
[0058] 4-Chlorobenzylamine (1.2 mmol) was added to 10 mL of DMF solution in which the intermediate thiophene-1,3,4-oxadiazole methyl ester (1.0 mmol) was dissolved, and the temperature was raised to 80 °C and reacted for 2 h. TLC monitored the reaction of the raw materials. , the system was cooled to room temperature, poured into ice water (50 mL), a solid was precipitated under stirring, filtered with suction, and the crude product was recrystallized from petroleum ether / ethanol mixed solvent after drying N-(4-chlorobenzyl)-5-thiophene-2 -yl-[1,3,4]oxadiazole-2-carboxamide (I-1): white solid; yield, 53.3%, m.p.183.6-185.9°C; 1H NMR (600MHz, DMSO-d6) δ9. 91(t,J=6.1Hz,1H),8.03(d,J=4.9Hz,1H),7.93(d,J=3.6Hz,1H),7.40(q,J=8.6Hz,4H),7.33( t, J=4.0Hz, 1H), 4.48 (d, J=6.2Hz, 2H). 13C NMR (150MHz, DMSO-d6) δ161.36, 157.78, 153.04, 137.38, 132.69, 131.62, 131.42, 129.33, 128.95, 128.26 ,123.58,41.89.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com