Preparation method and application of camphor sulfonyl benzylamine compound
A technology of sulfonylbenzylamine and compound, which is applied in the field of preparation of camphorsulfonylbenzylamine compounds, can solve the problems such as unreported preparation method and sterilization application of camphorsulfonylbenzylamine compound, camphor can not meet the market demand of camphor, and the like, Achieve the effect of novel molecular structure, simple preparation method, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
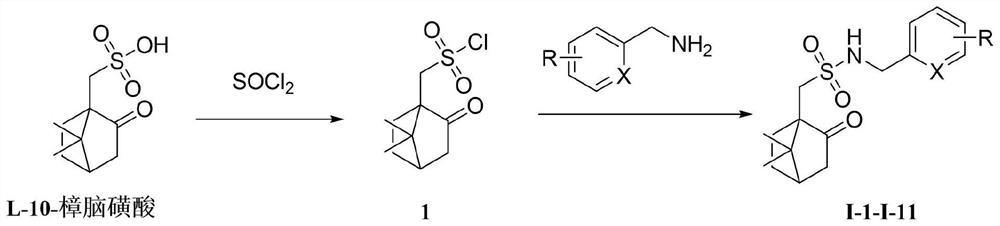
[0032] Add L-10-camphorsulfonic acid (3.0mmol) and thionyl chloride (3.3mmol) to the single-necked bottle to dissolve, heat up to reflux temperature for 5h, cool, concentrate to remove most of the solvent and HCl, and obtain white solid L-10 -camphorsulfonyl chloride, the product was directly put into the next step without further treatment.
[0033]
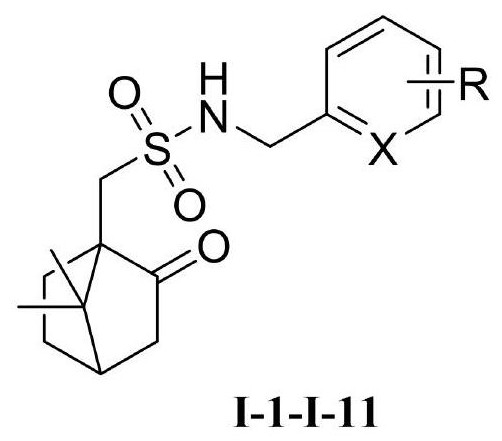
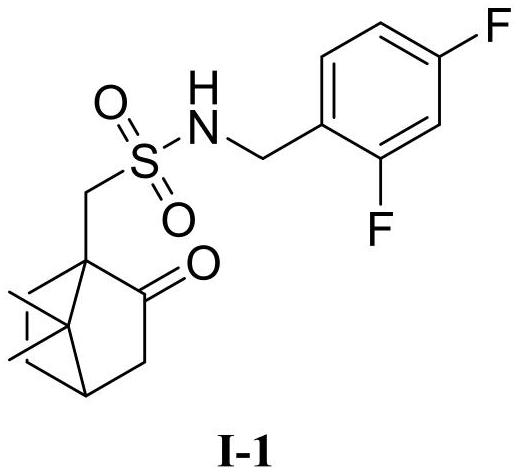
[0034] Dissolve 2,4-difluorobenzylamine (1.0mmol), 4-dimethylaminopyridine (0.1mmol), and triethylamine (1.1mmol) in anhydrous dichloromethane, cool to 0°C, and add L -10-Camphorsulfonyl chloride (1.10mol), raised to 25°C for 3h. The complete reaction of raw materials was monitored by TLC. The organic layer was washed 3 times with water (8ml×3), washed 3 times with saturated brine (8mL×3), dried, filtered with suction, concentrated to remove dichloromethane, and the crude product was subjected to column chromatography (CH 2 Cl 2 :MeOH=20:1) to obtain target compound I-1, white solid, m.p.52-54 ℃, yield 85%. 1 H NMR (600MHz...
Embodiment 2
[0036]
[0037] Dissolve 3-chlorobenzylamine (1.0mmol), 4-dimethylaminopyridine (0.1mmol), and triethylamine (1.1mmol) in anhydrous dichloromethane, cool to 0°C, and add L-10- Camphorsulfonyl chloride (1.10mol) was raised to 25°C for 5h. The complete reaction of raw materials was monitored by TLC. The organic layer was washed 3 times with water (8ml×3), washed 3 times with saturated brine (8mL×3), dried, filtered with suction, concentrated to remove dichloromethane, and the crude product was subjected to column chromatography (CH 2 Cl 2 :MeOH=20:1) to obtain target compound I-2, colorless oil, yield 80%. 1 H NMR (600MHz, CDCl 3)δ7.38(s,1H),7.28–7.27(m,3H),5.88(s,1H),4.37–4.29(m,2H),3.18(d,J=15.1Hz,1H),2.89(d ,J=15.1Hz,1H),2.39–2.35(m,1H),2.17–2.10(m,2H),2.04–1.90(m,3H),1.45–1.40(m,1H),0.96(s,3H) ),0.79(s,3H). 13 C NMR (150MHz, CDCl 3 )δ217.18, 139.11, 134.49, 129.95, 128.26, 127.92, 126.34, 59.27, 50.69, 48.79, 47.05, 42.91, 42.68, 26.94, 26.85, 19.71, 19.30.
Embodiment 3
[0039]
[0040] Dissolve 2-chloro-4-fluorobenzylamine (1.0mmol), 4-dimethylaminopyridine (0.1mmol), and triethylamine (1.1mmol) in anhydrous dichloromethane, cool to 0°C, and add in batches L-10-camphorsulfonyl chloride (1.10mol), raised to 25°C for 4.5h. The complete reaction of raw materials was monitored by TLC. The organic layer was washed 3 times with water (8ml×3), washed 3 times with saturated brine (8mL×3), dried, filtered with suction, concentrated to remove dichloromethane, and the crude product was subjected to column chromatography (CH 2 Cl 2 :MeOH=20:1) to obtain target compound I-3, white solid, m.p.106-107.5°C, yield 83%. 1 H NMR (600MHz, CDCl 3 )δ7.54(dd, J=8.5,6.0Hz,1H),7.16(dd,J=8.4,2.6Hz,1H),7.04–7.01(m,1H),5.91–5.89(m,1H),4.49 –4.42(m,2H),3.21(d,J=15.1Hz,1H),3.00–2.86(m,1H),2.42–2.38(m,1H),2.20–2.13(s,2H),2.06–2.00 (m,2H),1.94(d,J=18.6Hz,1H),1.49–1.43(m,1H),1.00(s,3H),0.83(s,3H). 13 C NMR (150MHz, CDCl 3 )δ216.72, 161.98(d, J=248.8Hz), 134.22(d, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com