Preparation method of thiophene-1, 3, 4-oxadiazole carboxamide compound, product and application thereof
A technology of oxadiazole carboxamide and thiophene carboxyl hydrazide is applied in the field of pesticide synthesis and achieves the effects of easy availability of raw materials, simple preparation method and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
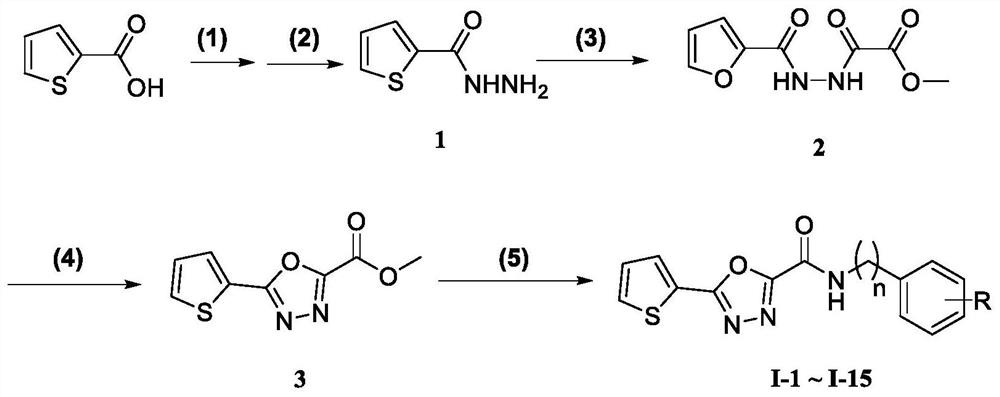
[0043] Schematic view of the preparation method of thiophene 1,3,4-malodazoleamide compounds of the present invention figure 1 , Including the following steps:
[0044] (1) 2-thiochloric acid was esterified by esterification of 2-thiophene;
[0045] (2) 2-thiophene carbide for a hydrazine, 2-thiophene hydrazide (1);
[0046] (3) 2-thiophene hydrazide and oxalyl monochloride reactively forms a bisamide intermediate (2);
[0047] (4) Disoxamide intermediate (2) is reacted with trichloroside, resulting in thiophene 1,3,4-malodoxazole (3);
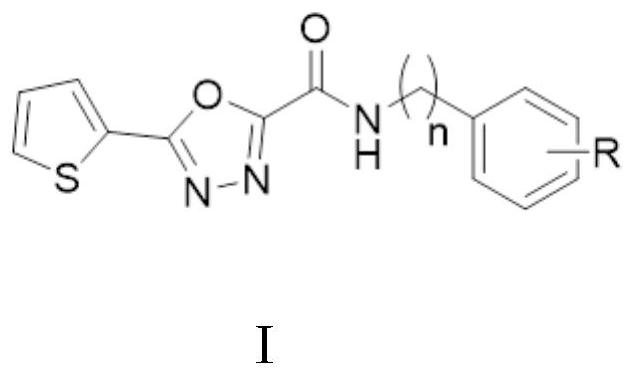
[0048] (5) thiophene 1,3,4-oxazoleate and the substituted benzylamine reaction synthesis thiophene 1,3,4-malodazoleamamide.
Embodiment 1
[0050] Preparation of thiophene 1,3,4-malodazole ester
[0051] 2-thiochloric acid (6.41 g, 50 mmol) was added to 250 mL of single-mouth bottle, and 100 mL of methanol and catalytic amount of concentrated sulfuric acid (0.5 g, 5 mmol) were added, increased to reflow temperature, reaction for 6 h, TLC detection, reaction, cooling To room temperature, the saturated sodium hydrogencarbonate solution was added dropwise to pH = 7, and ethyl acetate was added (50 ml × 3), combined with the organic layer, washed with brine (100 ml × 2), dried over anhydrous sodium sulfate. Filtration, dissolve the oil 6g.
[0052]6 g of the 6 g of the oil was dissolved in 60 ml of ethanol, and 85% hydrazine (7.94 g, 211 mmol) was added, and the reflux reaction was 5 h, the TLC detection raw material reaction was complete, cooled, rotated to remove most of ethanol, precipitate solid, and filtered Wash (10 mL × 3), dried 2-thiophene hydrazide.
[0053] 2-thiophene hydrazide (0.99 g, 7.0 mmol) was dissolved...
Embodiment 2
[0056] Preparation of thiophene 1,3,4-oxazoleamide
[0057]
[0058] 4-chlorobenzylamine (1.2 mmol) was added to a solution of an intermediate thiophene 1,3,4-oxagnectate (1.0 mmol) 10 ml of DMF, temperature rising to 80 ° C reaction for 2 h, TLC monitoring the feedstock reaction The system is cooled to room temperature, poured into ice water (50 mL), stirring, stirring solid, filtration, drying after drying, crystallization N- (4-chlorophenyl) -5-thiophene-2 - Base - [1,3,4] malodazole-2-formamide (I-1): white solid; yield, 53.3%, MP183.6-185.9 ° C; 1H NMR (600 MHz, DMSO-D6) Δ9. 91 (t, j = 6.1Hz, 1H), 8.03 (D, J = 4.9 Hz, 1H), 7.93 (D, J = 3.6Hz, 1H), 7.40 (Q, J = 8.6Hz, 4H), 7.33 ( T, J = 4.0Hz, 1H), 4.48 (D, J = 6.2 Hz, 2H) .13C NMR (150MHz, DMSO-D6) δ161.36, 157.78, 153.04, 137.31.42, 129.31.62, 131.42, 129.33, 128.95, 128.26 123.58, 41.89.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com