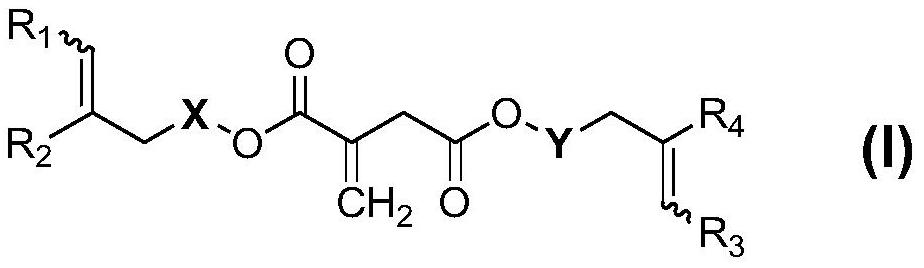
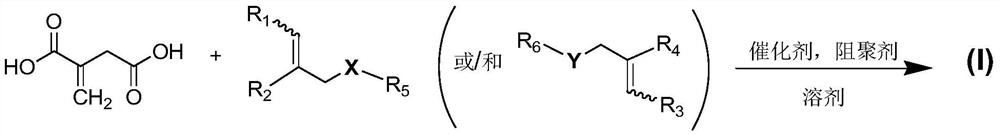
Itaconic acid diester type photocuring monomer, composition, preparation method and application
A technology of itaconate diester and light-curing monomer, which is applied in the preparation of organic compounds, carboxylic acid ester preparation, chemical instruments and methods, etc., to achieve low volatility, good mutual solubility and copolymerization ability, and strong application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Utilize 3-bromo-2-methylpropene to prepare target product monomer (I)-1
[0043]
[0044] Add 26.0 grams (0.2mol) of itaconic acid, 55.2 grams (0.4mol) of potassium carbonate and 250 milliliters of DMF into a 500ml three-necked flask, then add 0.01mol of hydroquinone for polymerization inhibition, heat to 70°C, and stir for 30 minutes. 53.6 g (0.4 mol) of 3-bromo-2-methylpropene was added dropwise into the reaction system, stirring was continued for 12 hours, and the reaction was monitored by TLC to complete. Inorganic salts were filtered off and most of the organic solvents were distilled off. Dichloromethane, extracted with deionized water, dried, evaporated to dryness, and distilled under reduced pressure to obtain 40.7 g of the product. Yield 85.5%. GC monitored purity 99%.
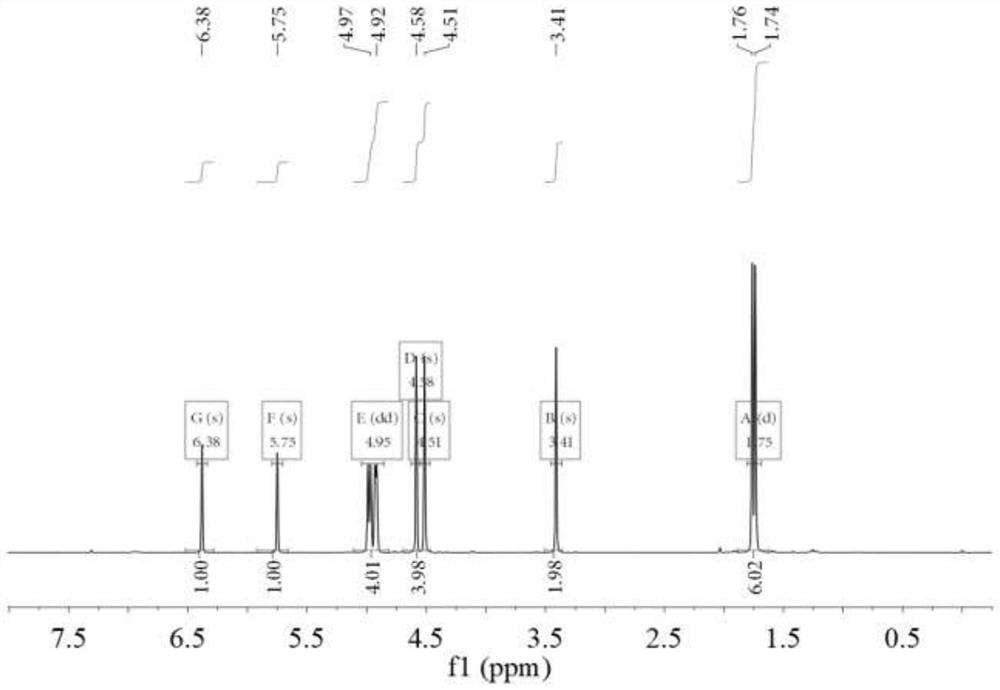
[0045] 1 H NMR (CDCl 3 ,400MHz),δ6.38(s,1H,=CH 2 ),5.75(s,1H,=CH 2 ),4.97(d,1H,=CH 2 ),4.92(d,2H,=CH 2 ),4.58(s,2H,CH 2 ),4.51(s,2H,CH 2 ),3.41(s,2H,CH 2 ),1.76(s,3...
Embodiment 2
[0047] Embodiment 2: Utilize 2-methallyl alcohol to prepare target product monomer (I)-1
[0048]
[0049] Add 26.0 grams (0.2mol) of itaconic acid and 250 milliliters of toluene in a 500 milliliter three-necked flask, then add 0.01mol hydroquinone as a polymerization inhibitor, add a reflux condenser and a water separator, and add 34.6 grams dropwise under heating and reflux (0.48mol) 2-methallyl alcohol into the reaction system, continue to reflux for 12 hours, TLC monitors the end of the reaction. Washed three times with deionized water, dried over anhydrous sodium sulfate, rotary evaporated toluene and excess 2-methallyl alcohol, and vacuum distilled to obtain 42.7 g of the target product. Yield 89.7%. GC monitored purity 99%.
Embodiment 3
[0050] Embodiment 3: Utilize 3-methyl-3-buten-1-alcohol to prepare target product body (I)-2
[0051]
[0052] Add 26.0 grams (0.2mol) of itaconic acid and 250 milliliters of toluene in a 500 milliliter three-necked flask, then add 0.01mol hydroquinone as a polymerization inhibitor, add a reflux condenser and a water separator, and add 41.3 grams dropwise under heating and reflux (0.48mol) 3-methyl-3-buten-1-ol into the reaction system, and continued to reflux for 18 hours, and TLC monitored the end of the reaction. Washed three times with deionized water, dried over anhydrous sodium sulfate, rotary evaporated toluene and excess 3-methyl-3-buten-1-ol, and vacuum distilled to obtain 45.5 g of the target product. Yield 85.6%. GC monitored purity 99%.
[0053] HR-MS (C 15 h 22 o 4 ): m / e: 266.15; Experimental result: 267.16 (M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com