Novel dimeric alkaloid as well as preparation and application thereof
A dimerized, alkaloid technology, applied in the field of alkaloids, can solve the problems of interfering with normal physiological functions, affecting gene expression and regulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Extraction and separation of novel alkaloids
[0033] 1.1 Experimental medicinal materials:
[0034] Bitter bean fruit was provided by Zhengzhou Zhongguan Jianye Biotechnology Co., Ltd.
[0035] 1.2 Extraction and separation of new alkaloids from the seeds of Sophora aphorae:
[0036] Get 50Kg of bitter bean seeds and grind into powder for subsequent use. Use 75% ethanol as the solvent to heat and reflux at 85°C for three times, each time for 2.5 hours, the ratio of solid to liquid is 1:10, and combine and concentrate the filtrate to obtain the medicinal solution for later use.
[0037] Dissolve the extracted medicinal liquid with as little water as possible, adjust the pH to 10.5 with sodium hydroxide solution, and the volume ratio of the aqueous solution dissolved in chloroform and medicinal liquid is 1:1, place it in a separatory funnel, shake it, and let it stand until the layers are separated Clear, the chloroform part is in the lower layer, take t...
Embodiment 2
[0041] Example 2: Investigate the structural identification and analysis of new alkaloid compounds
[0042] The novel alkaloid compound that above-mentioned embodiment 1 obtains carries out structural test analysis, obtains following test data:
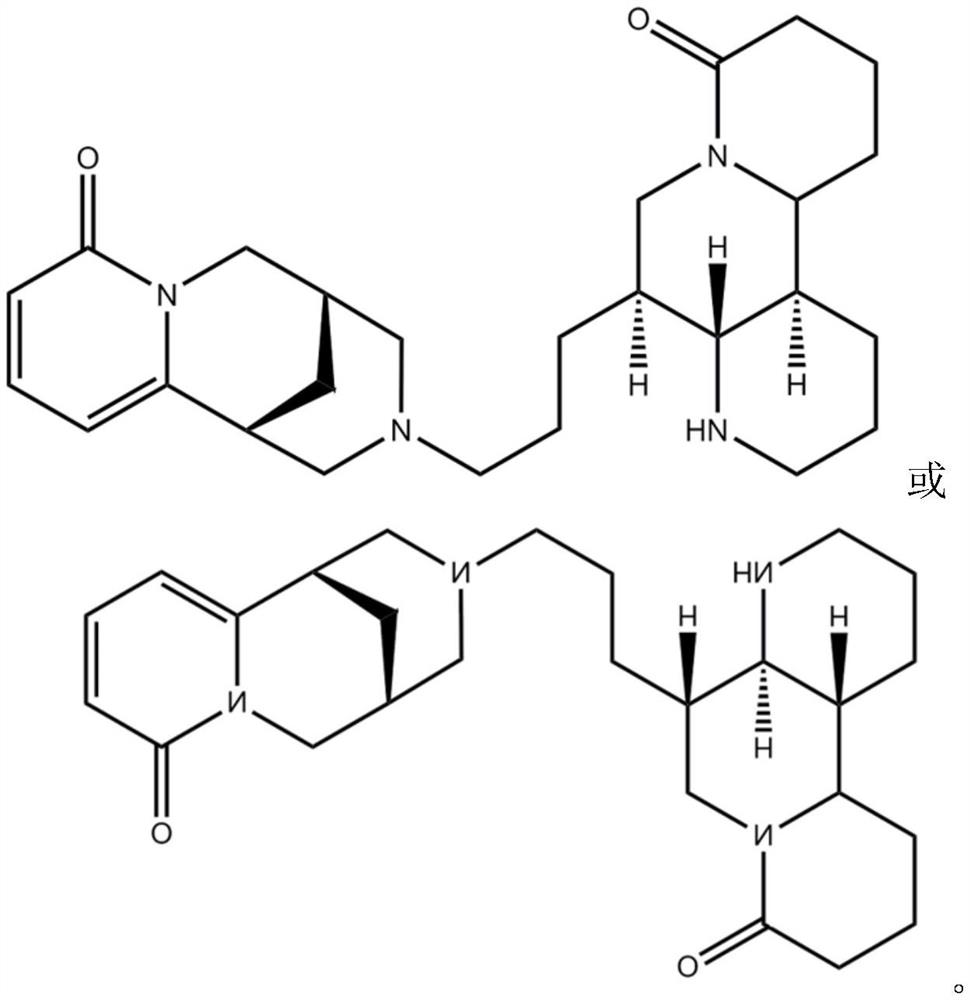
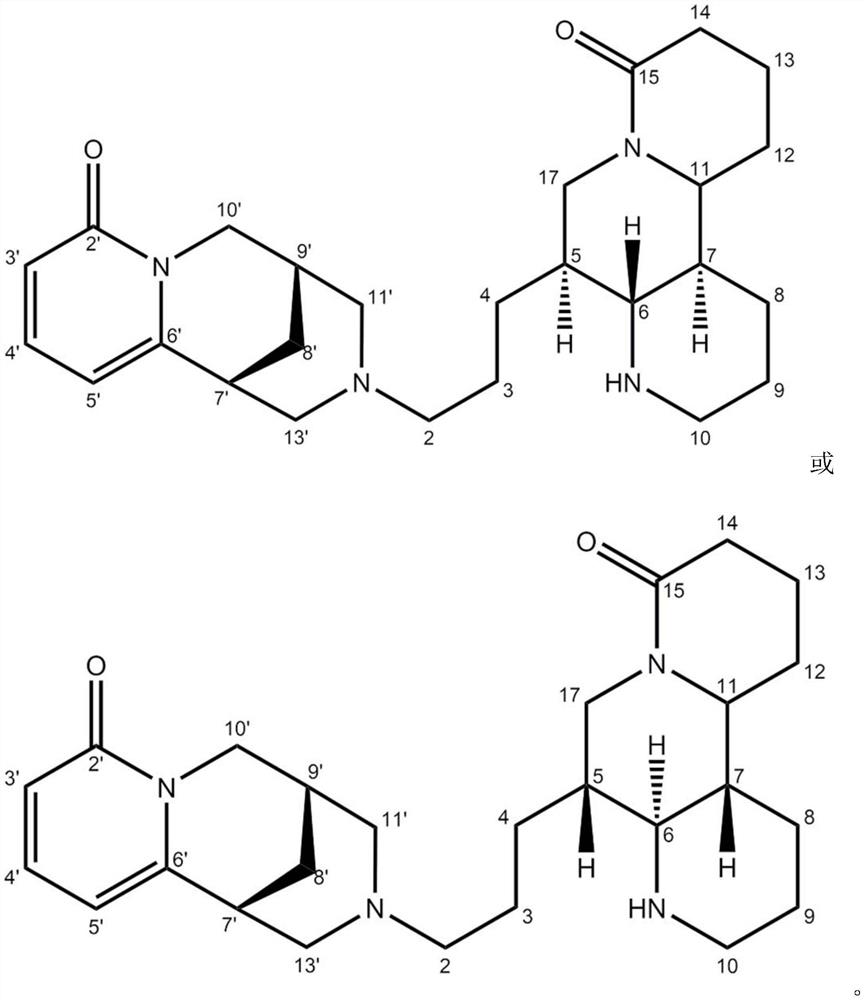
[0043] Formula I compound: C 26 h 38 N 4 o 3 It is light yellow amorphous powder, slightly soluble in DMSO. HR-ESI-MS (m / z439.3058[M+H] + , calculated value 439.3073). Through HSQC and HMBC spectrum, combined with 1 H NMR and 13The information given by the C NMR spectrum assigns the hydrocarbon information of the above-mentioned new compounds, as shown in Table 1. In the HMBC spectrum, δ H 7.39(H-4') and δ C 151.6(C-6'), δ C 162.1 (C-2') correlation, δ H 6.22(H-3') and δ C 104.0(C-5’) related, δ H 5.76(H-5') and δ C 34.1(C-7'), δ C 115.1(C-3'), δ C 151.6 (C-6') correlation, δ H 3.76(H-10') and δ C 25.6(C-8'), δ C 27.3(C-9'), δ C 57.6 (C-11') correlation, δ H 2.68(H-2) and δ C 57.6 (C-11') correlatio...
Embodiment 3
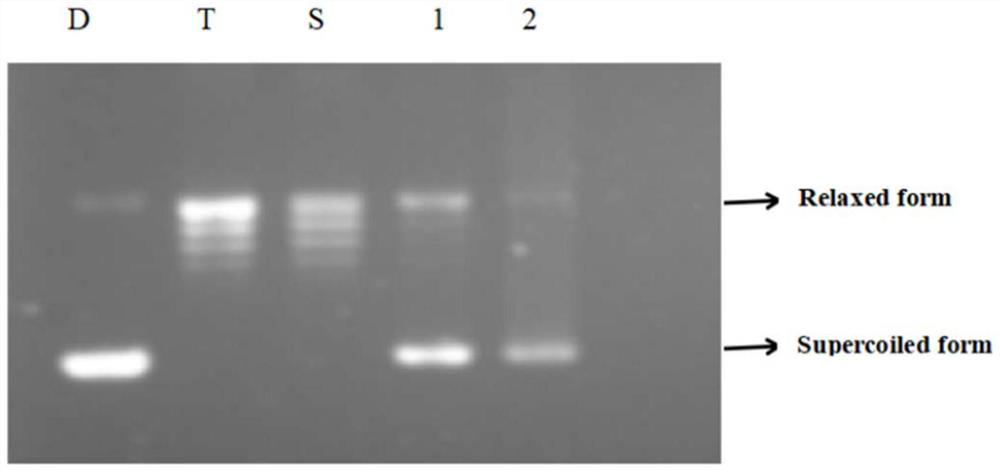
[0053] Embodiment 3: the inhibition Topo I activity experiment of novel alkaloid compound
[0054] 3.1 Experimental materials:
[0055] The new alkaloids used in the experiment were prepared by the method of the above-mentioned Example 1, weighed accurately, dissolved and diluted with DMSO to a concentration of 1 mM. EDTA (Shanghai Adamas Reagent Co., Ltd.); EB (Sigma-Aldrich Trading Co., Ltd.); Agarose (Shanghai Adamas Reagent Co., Ltd.); pBR322DNA (Baoric Medical Biotechnology Co., Ltd.); DNA topoisomerase I (Baoriyi Biotechnology Co., Ltd.); DNA Loading Buffer (6×) (Shanghai Biyuntian Biotechnology Co., Ltd.).
[0056] 3.2 Experimental method:
[0057] (1) Preparation of 1% agarose gel:
[0058] Accurately weigh 0.5g of agarose powder, dissolve it with 50mL of 1×TAE buffer at high temperature, cool to 60°C and pour it into the horizontal tank of gel electrophoresis.
[0059] (2) Preparation of 1×Topo I Buffer:
[0060] Pipette 10 μL of 10×Topo I Buffer and dilute with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com