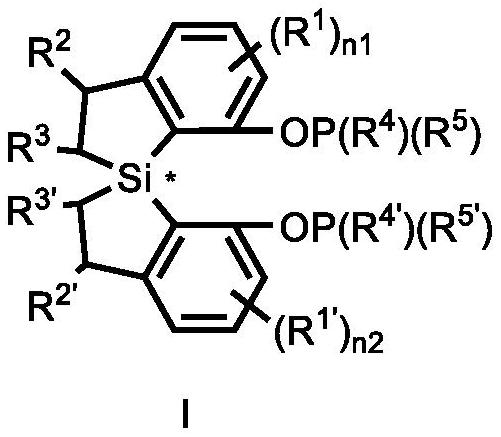
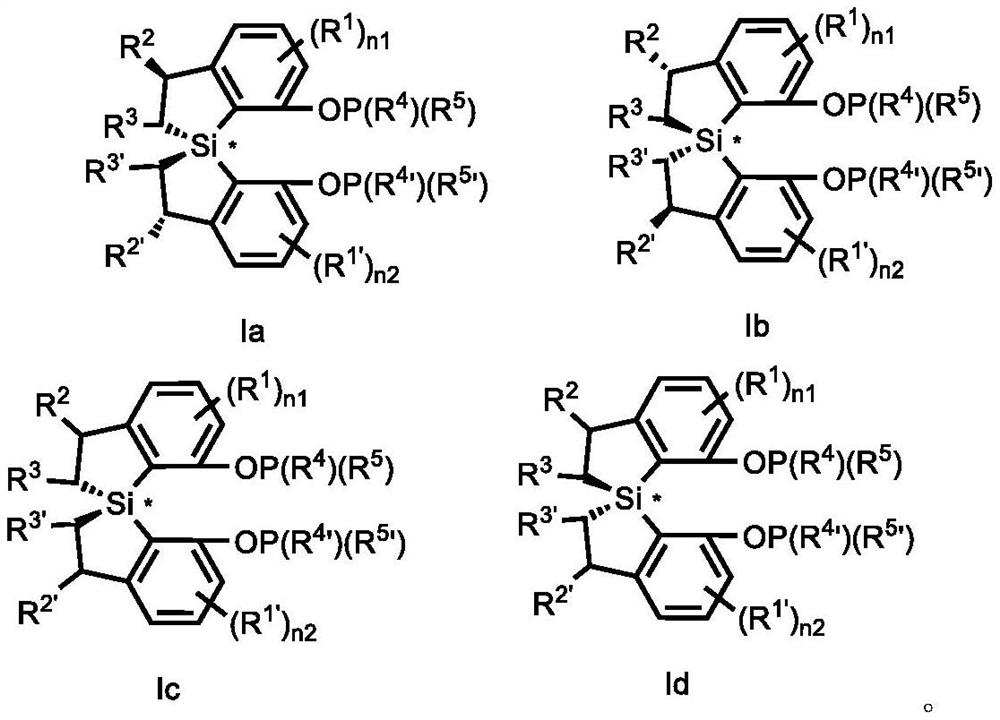
Spiro bis (dihydrobenzosilole) phosphonite monoester compound as well as preparation and application thereof
A compound and single-bond technology, applied in the field of spirobisdihydrobenzosilrole phosphonite monoester compounds, can solve the problem of single phosphonite monoester ligands and achieve good enantioselectivity and catalytic High activity, product enantioselectivity, high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
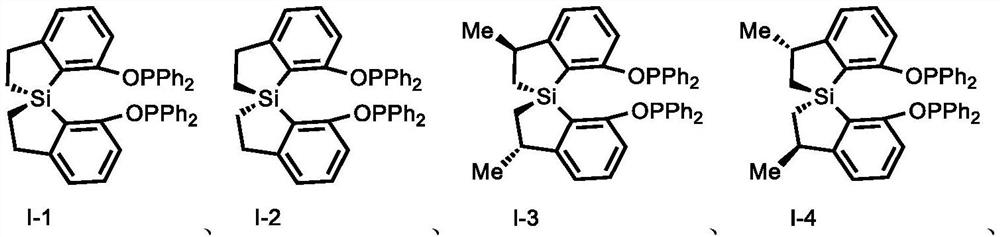
[0158] Example 1: I-4
[0159]
[0160] Under a nitrogen atmosphere, in a 10 mL dry Schlenk tube, add spirobisdihydrobenzothiazole diol (296.4 mg, 1.0 mmol) and dissolve in 2.0 mL of tetrahydrofuran, add TEA (0.7 mL, 5.0 mmol), room temperature Slowly add diphenylphosphorus chloride (0.7mL, 4.0mmol) under stirring, TLC detects that the reaction is complete, filter with diatomaceous earth, wash with ethyl acetate, and the filtrate is desolventized by a rotary evaporator and purified by column chromatography to obtain the spirochete Bisdihydrobenzosilrole phosphite (531.8 mg, yield: 80%).
[0161]
[0162] white solid, [a] D 24 =+142.87 (c=0.90, CHCl 3 ); 1 H NMR (400MHz, acetone-d 6 )δ7.43–7.32(m,12H),7.17(q,J=3.9Hz,2H),7.09(ddd,J=12.4,6.7,4.7Hz,12H),3.32–3.18(m,2H),1.61 (dd,J=15.1,8.4Hz,2H),1.24(d,J=7.0Hz,6H),0.81(dd,J=15.1,4.0Hz,2H). 31 P NMR (162MHz, acetone-d 6 )δ102.02. HRMS (ESI-TOF) m / z calculated value C 42 h 38 o 2 P 2 Si[M+H] + :665.2116, test value...
Embodiment 2
[0163] Example 2: I-3
[0164]
[0165] White solid, 77% yield, [a] D 24 =-142.87 (c=0.90, CHCl 3 ); 1 H NMR (400MHz, acetone-d 6 )δ7.43–7.32(m,12H),7.17(q,J=3.9Hz,2H),7.09(ddd,J=12.4,6.7,4.7Hz,12H),3.32–3.18(m,2H),1.61 (dd,J=15.1,8.4Hz,2H),1.24(d,J=7.0Hz,6H),0.81(dd,J=15.1,4.0Hz,2H). 13 C NMR (100MHz, chloroform-d) δ162.18, 154.17, 132.78, 127.72, 122.97, 120.21, 40.12, 25.52, 5.96; 31 P NMR (162MHz, acetone-d 6 )δ102.02. HRMS (ESI-TOF) m / z calculated value C 18 h 20 o 4 SiP[M+H] + : 359.0863, test value: 359.0863. HRMS (ESI-TOF) m / z calculated value C 42 h 38 o 2 P 2 Si[M+H] + :665.2116, test value: 665.2196.
Embodiment 3
[0166] Example 3: I-1
[0167]
[0168] White solid, 87% yield, [a] D 24 =-159.87 (c=0.90, CHCl 3 ); 1 H NMR (400MHz, acetone-d 6 )δ7.35(dt, J=10.9,7.3Hz,12H),7.22–7.06(m,12H),6.99(d,J=7.5Hz,2H),3.00(dddd,J=31.5,17.1,9.5, 5.3Hz, 4H), 1.29(ddd, J=15.6, 9.7, 6.0Hz, 2H), 1.14(ddd, J=14.9, 9.3, 4.7Hz, 2H).; 13 C NMR (101MHz, acetone-d 6 )δ161.02,160.93,157.07,140.93,140.75,140.62,140.45,132.19,132.17,130.74,130.50,129.93,129.37,129.15,129.00,128.47,128.40,128.36,128.29,126.35,120.16,112.32,112.09,31.43,8.69 . 31 P NMR (162MHz, acetone-d 6 )δ101.02. HRMS (ESI-TOF) m / z calculated value C 40 h 34 o 2 P 2 Si[M+H] + :637.1803, test value: 637.1859.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com