Bipyrazine macrocyclic compound, preparation method and application of bipyrazine macrocyclic compound in construction of fluorescent powder
A technology of macrocyclic compounds and phosphors, which is applied in the cross field of organic materials and coordination chemistry, can solve the problems of high cost, insufficient supply, complex synthesis steps, etc., and achieve the effect of low cost, low industrial cost and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Synthesis of double pyrazine macrocyclic compound 1,3-OBP
[0047]
[0048]Under nitrogen atmosphere, 1,3-dihydroxynaphthalene (1.60g, 10mmol), 2,6-dichloropyrazine (1.49g, 10mmol), cesium carbonate (6.5g, 20mmol) were added to DMSO (100mL) in a round bottom flask. After vigorously stirring at 120° C. for 18 h, the reaction mixture was poured into 200 mL of water, extracted three times with 100 mL of ethyl acetate, and the organic phase was recovered. Wash the organic phase with saturated brine, reclaim the organic phase; wash with anhydrous Na 2 SO 4 The organic phase was dried, filtered and concentrated in vacuo to give crude product. Using petroleum ether and ethyl acetate (volume ratio of 5:1) as a developing solvent, the crude product was purified by column chromatography (using a 200-mesh silica gel column) to obtain the bispyrazine macrocyclic compound 1,3- OBP, yield: 51.7% (calculated as 2,6-dichloropyrazine).
[0049] 1,3-OBP (C 28 h 16...
Embodiment 2
[0056] Embodiment 2: Synthesis of double pyrazine macrocyclic compound 2,3-OBP
[0057]
[0058] The preparation method of 2,3-OBP is the same as that of 1,3-OBP, except that 1,3-dihydroxynaphthalene is replaced by 2,3-dihydroxynaphthalene (1.60g, 10mmol), yield: 23.1% (with 2, 6-dichloropyrazine calculation).
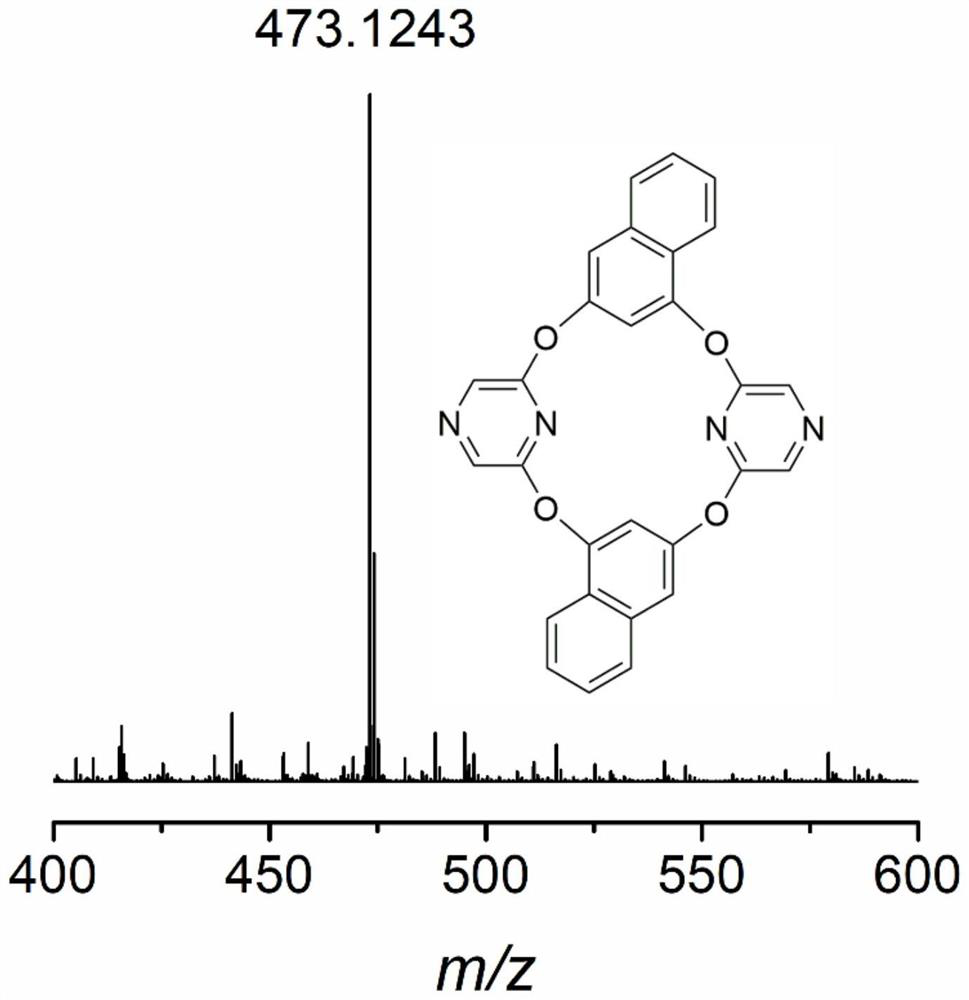
[0059] 2,3-OBP (C 28 h 16 N 4 o 4 ), theoretical value of high resolution mass spectrometry: [M+H] + :473.1172, experimental value: 473.1242( Figure 8 ). Elemental analysis theoretical value: C 71.18%; H 3.41%; N 11.86%, experimental value: C 70.91%; H 3.52%; N 31.66%.
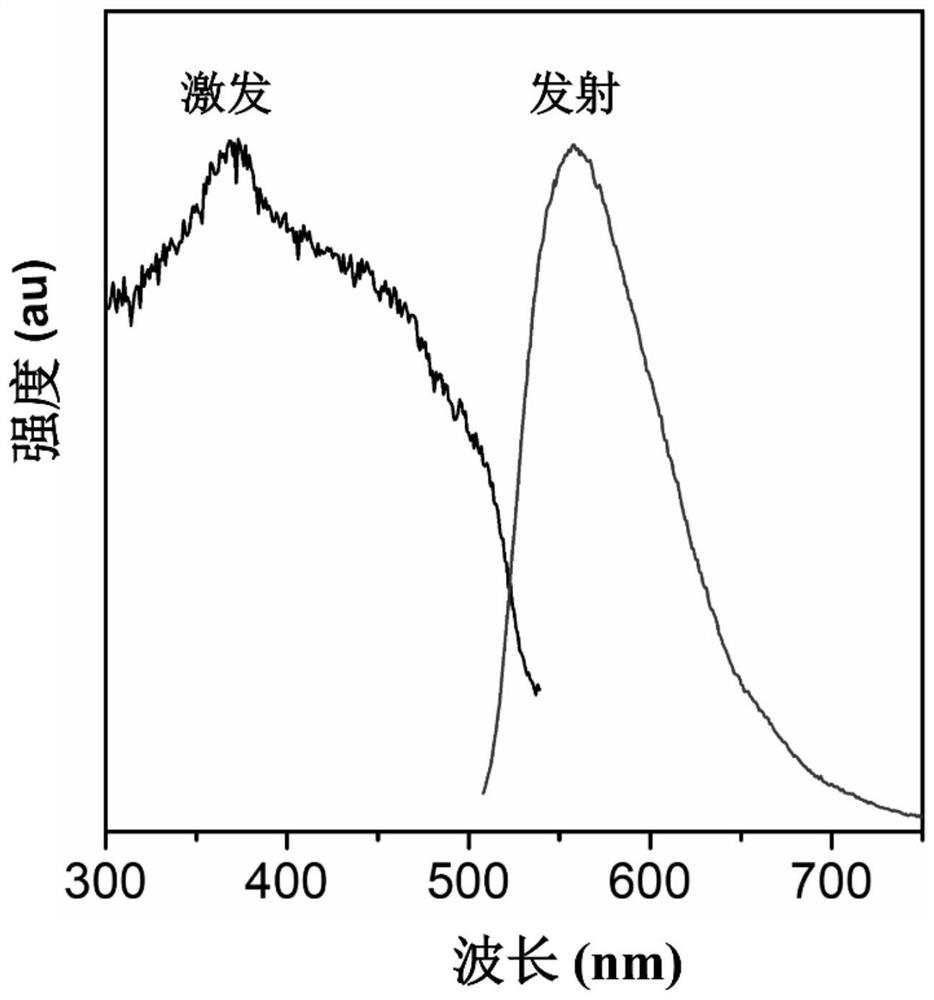
[0060] The phosphor powder with high luminous efficiency is prepared by using the above bispyrazine macrocyclic compound 2,3-OBP. 2,3-OBP (4.7mg, 0.01mmol) and cuprous iodide (3.8mg, 0.02mmol) were completely dissolved in acetonitrile (2mL), respectively, and the two clear solutions were mixed together, and placed in a dark room at room temperature for 1 days, the MOFs obtained after part ...
Embodiment 3
[0063] Embodiment 3: Synthetic double pyrazine macrocyclic compound OFP
[0064]
[0065] The preparation method of OFP is the same as that of 1,3-OBP, except that 1,3-dihydroxynaphthalene is replaced by fluorescein (3.32g, 10mmol), the yield: 19.3% (calculated as 2,6-dichloropyrazine).
[0066] OFP(C 48 h 24 N 4 o 10 ), theoretical value of high resolution mass spectrometry: [M+H] + :817.1492, experimental value: 817.0638( Figure 14 ). Elemental analysis theoretical value: C 70.59%; H 2.96%; N 6.86%, experimental value: C 70.37%; H 3.11%; N 6.49%.
[0067] The phosphor powder with high luminous efficiency is prepared by using the bispyrazine macrocyclic compound. OFP (8.2mg, 0.01mmol) and cuprous iodide (3.8mg, 0.02mmol) were completely dissolved in acetonitrile (2mL), respectively, and the two clear solutions were mixed together, left standing in a dark room at room temperature for 1 day, and part of the solvent After volatilization, MOFs 3( Figure 15 is the sc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com