Preparation method and application of anthraquinone ester-based fragrance-fixing slow-release agent
An anthraquinone and alkyl technology, applied in the field of ester compounds based on anthraquinone structure and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
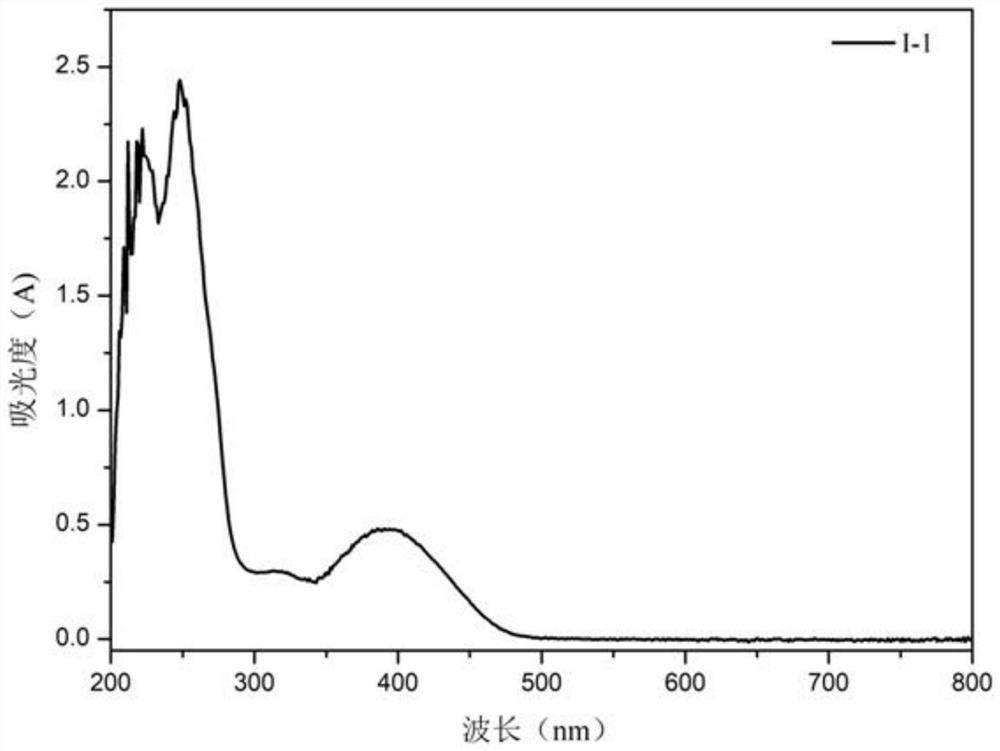
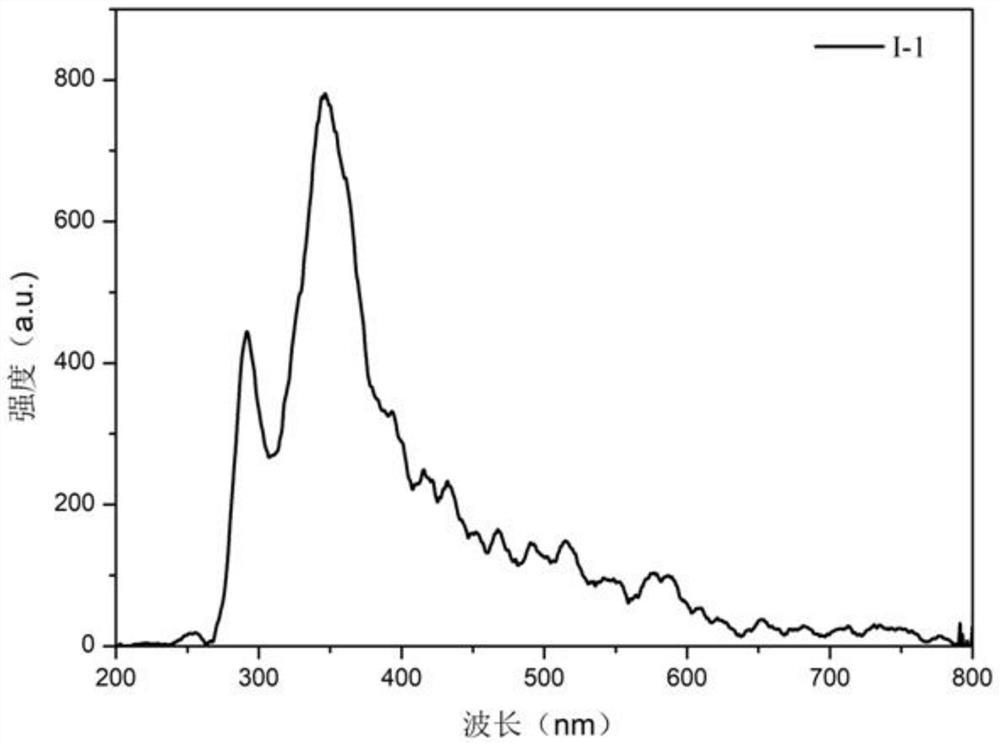
[0039] Compound I-1
[0040]
[0041] (1) Preparation of Intermediate 6-1
[0042] 1-phenylethyl alcohol compound 5-1 (3.66 g, 30 mmol) and pyridine (2.37 g, 30 mmol) were added to 15 ml of dichloromethane, and the bromine bromide 4 (6.03 g, 30 mmol) was added dropwise at 0 ° C. 1H, gradually increased to room temperature, end the reaction after 2 h after 2 hours of room temperature, and extracted three times with dichloromethane, adding silica gel powder in the filtrate, and the column chromatography obtained -1 (eluent is petroleum ether: ethyl acetate = 20: 1).
[0043] The NMR of the intermediate 6-1 is as follows: 1 H NMR (400 MHz, ChlorOform-D) δ 7.28-7.11 (m, 5H), 5.85-5.75 (m, 1H), 3.67 (S, 2H), 1.45 (D, J = 6.6 Hz, 3H). 13 C NMR (101 MHz, Chloroform-D) δ166.46, 140.76, 128.66, 127.38, 126.20, 74.47, 26.35, 20.80.
[0044] (2) Preparation of Compound I-1
[0045]
[0046] 1,4-dihydroxy hydrazine compound 1 (0.6 g, 2.5 mmol) was added to 20 mL of acetonitrile, then cesi...
Embodiment 2
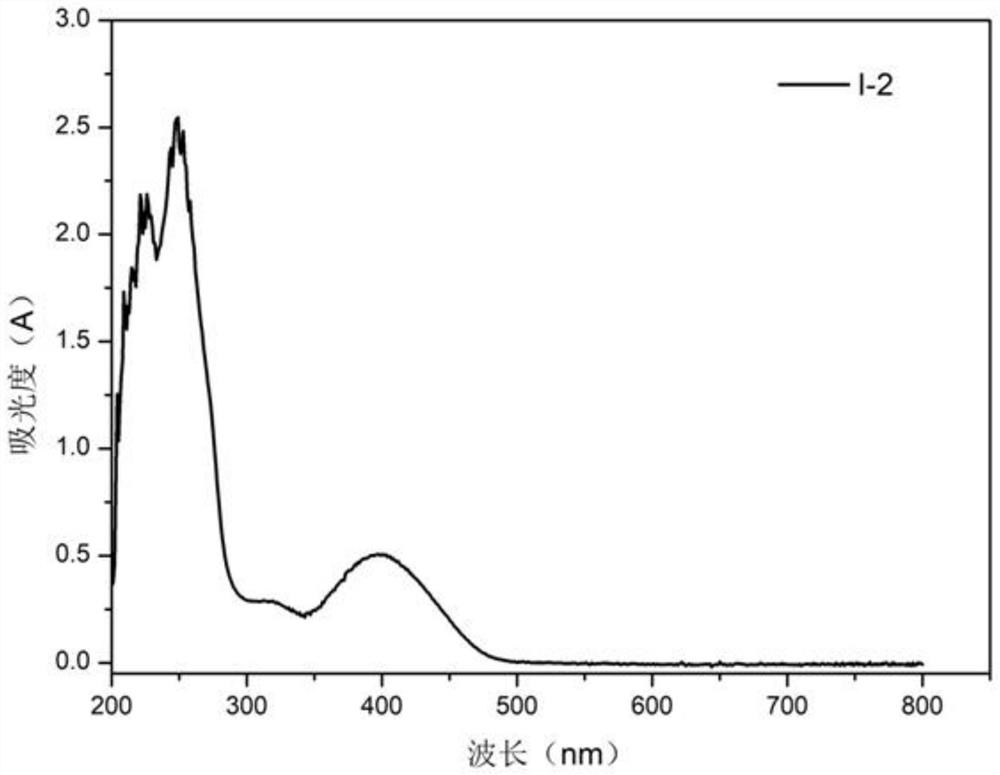
[0049] Compound I-2
[0050]
[0051] (1) Preparation of Intermediate 6-2
[0052]Dimethylbenylylbenyl alcohol compounds 5-2 (4.5 g, 30 mmol) and pyridine (2.37 g, 30 mmol) were added to 15 ml of dichloromethane, and the bromine bromide 4 (6.03) was added dropwise to 0 ° C. g, 30 mmol), reaction 1H, gradually increased to room temperature, end the reaction after 2 h at room temperature, dissolved the white solid, extracted three times with dichloromethane, adding silica gel powder in the filtrate, obtained by column chromatography Intermediate 6-2 (eluent is petroleum ether: ethyl acetate = 20: 1).
[0053] The NMR of Compound 6-2 is as follows: 1 H NMR (400 MHz, ChlorOform-D) δ 7.20-6.99 (m, 5H), 3.58 (S, 2H), 2.35 (S, 2H), 1.35 (S, 6H). 13 C NMR (101 MHz, Chloroform-D) δ166.29, 136.76, 130.68 (Q, J = 2.9 Hz), 128.41-127.87 (M), 126.73, 84.59, 46.50, 27.73, 25.68.
[0054] (2) Preparation of Compound I-2
[0055]
[0056] 1,4-dihydroxy hydrazine compound 1 (0.6 g, 2.5 mmol) w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com