Chemotherapy immune gel as well as preparation method and application thereof
A chemotherapy immune and gel technology, which is applied in the field of chemotherapy immune gel and its preparation, can solve the problems of large clinical side effects, etc., and achieve the effect of enhanced immunogenic tumor phenotype, good therapeutic effect, and strong systemic anti-cancer immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
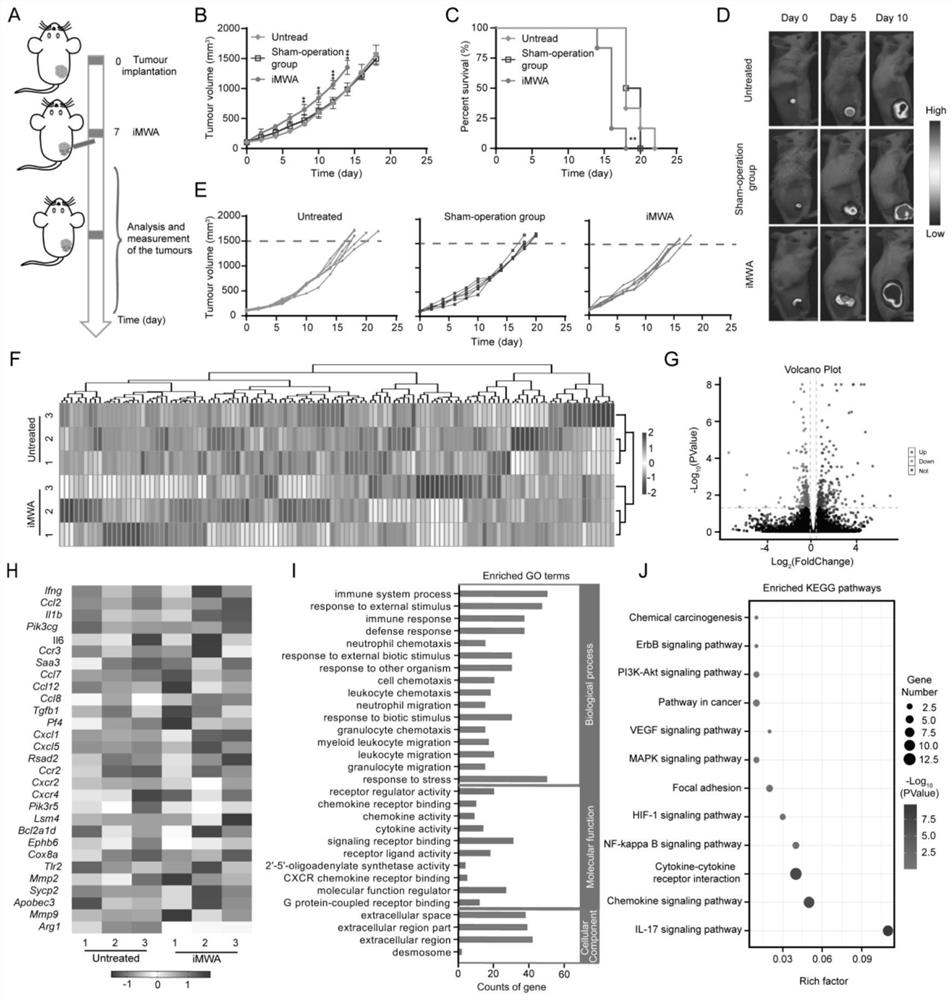
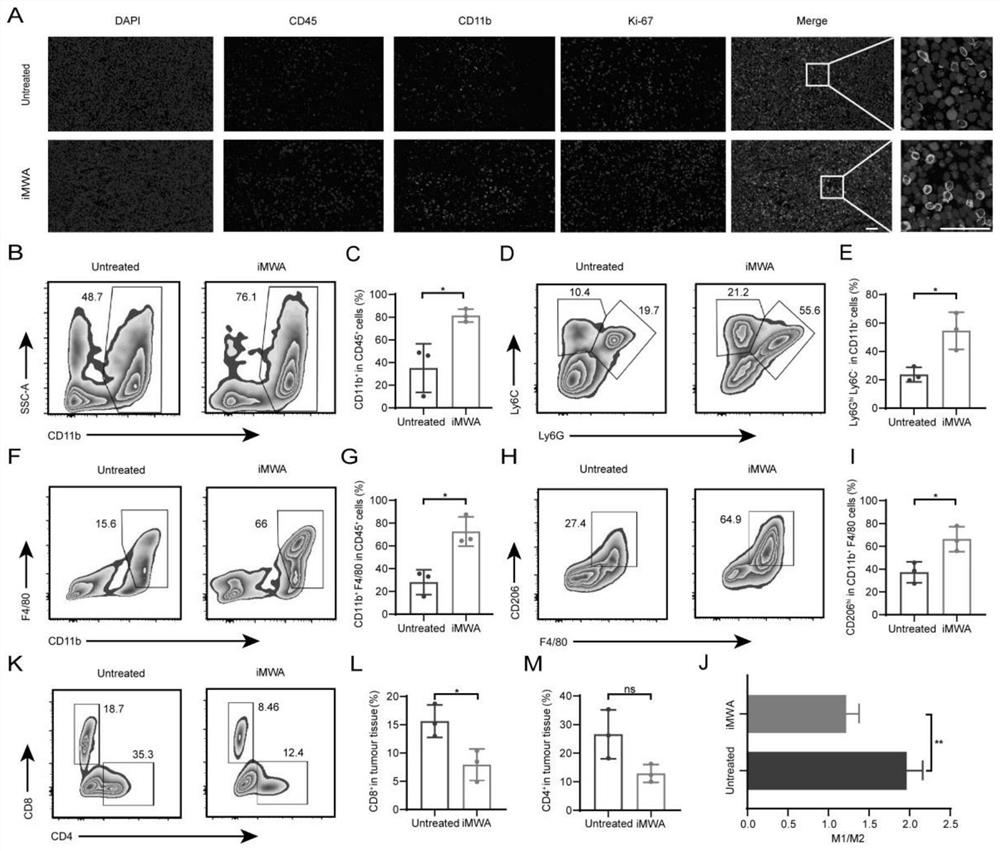
[0038] Example 1: Establishment and analysis of mouse model iMWA
[0039] The establishment of a mouse model iMWA accelerated tumor progression and induced immunosuppression. The establishment of the mouse model and the experimental process were as follows: BALB / c mice (6-8 weeks old) were inoculated with CT26 colorectal cancer cells (per mice were inoculated with 1x10 6 100 μL of PBS for each cell). When the tumor volume grows to about 100mm 3 Treatment was started at the time (7 days after tumor implantation), and randomized grouping and treatment were performed. A mouse model of incomplete ablation was established using a microwave therapeutic apparatus (ECO-100E, Nanjing Yigao Microwave Electric Research Institute, China). After anesthetizing CT26 tumor-bearing mice, a cold-tip MWA needle with a 1 cm active needle was placed percutaneously in the middle of the long axis of the tumor, and the power and time of ablation were controlled at 5 W and 1-1.5 min, respectively. ...
Embodiment 2
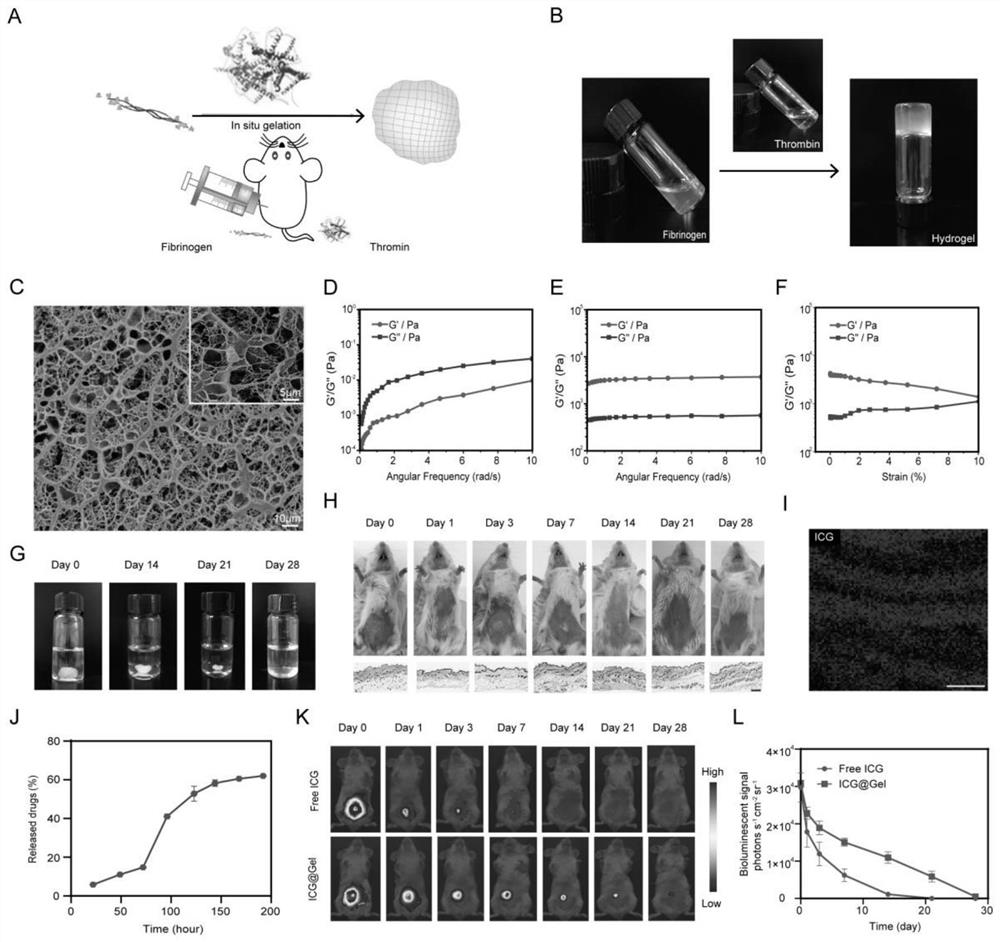
[0044] Example 2: Preparation and Identification of Chemotherapy Immunogel Drugs
[0045] In this embodiment, a chemotherapy immune gel drug is prepared, and its preparation method specifically includes the following steps:
[0046] The fibrinogen solution (50 mg / mL) was dissolved in deionized water or PBS and mixed under ultrasound for 3 minutes. The fibrinogen solution was then heated to 37°C for 30 minutes. Then thrombin (500IU / mL) was dissolved in deionized water or PBS (containing 26mg / L NaCl and 7mg / L CaCl 2 ). The drug-loaded gel was prepared by adding a predetermined amount of OX (final concentration 1 mg / mL) and IPI549 (final concentration 0.25 mg / mL) into the above-mentioned fibrinogen solution (in this step, when only OX or For IPI549, OX@Gel and IPI549@Gel for control experiments can be prepared respectively). Then the thrombin and fibrinogen solution were mixed at a volume ratio of 1:2 with a double-barreled syringe, and a fibrin gel (200 μL) was formed within...
Embodiment 3
[0052] Example 3: Study on the Treatment of Local Residual Cancer with Chemotherapy Immunogel
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com