Rupatadine fumarate intermediate and preparation method of rupatadine fumarate
A technology for rupatadine fumarate and an intermediate, which is applied in the field of chemical synthesis, can solve the problems of complicated post-processing, difficult to remove by-products, harsh reaction conditions, etc. Moderate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0074] Example 1 Compound V Preparation
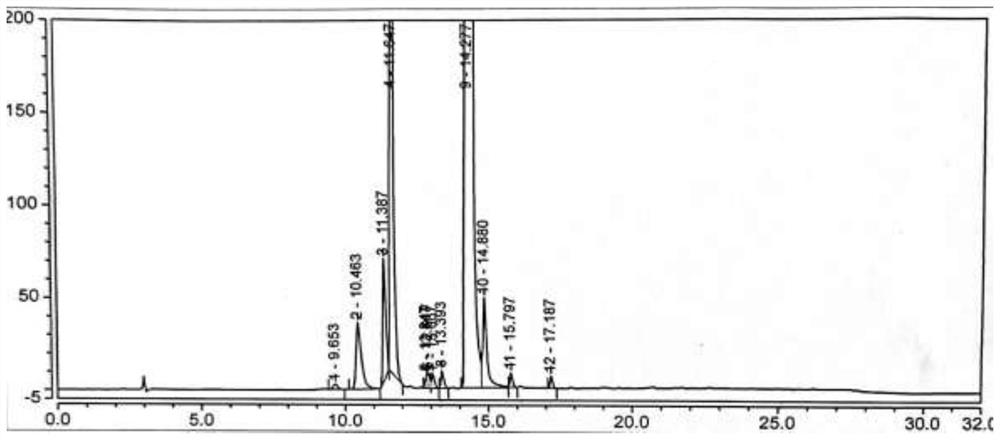
[0075] Under nitrogen protection, add the compound of formula (VI) (2.5g, 0.02mol, 1.0eq) into a 100ml reaction flask, add 50ml of acetonitrile, start stirring, heat up to reflux, add NCS (3.2g, 0.024mol, 1.2eq), AIBN (0.33g, 0.002mol, 0.1eq), reacted for 6h, TLC (developing solvent, methanol:dichloromethane=1:2, add 2 drops of ammonia) to monitor the disappearance of the compound of formula (Ⅵ), concentrated the acetonitrile under reduced pressure, added 20ml of toluene was stirred for 30min, filtered, and the filtrate was collected, and 60ml of n-hexane was added dropwise with stirring at room temperature. After the dropwise addition was completed, the temperature was lowered to 0-10°C and stirred for 60min, filtered, and dried to obtain the compound of formula (Ⅴ) (2.8g), with a yield of 88.9% , HPLC purity 86.05%, see figure 1 . MS-ESI(m / z): 157.1[M+H] + ,See Image 6 .
example 2
[0076] Preparation of Example 2 Compound V
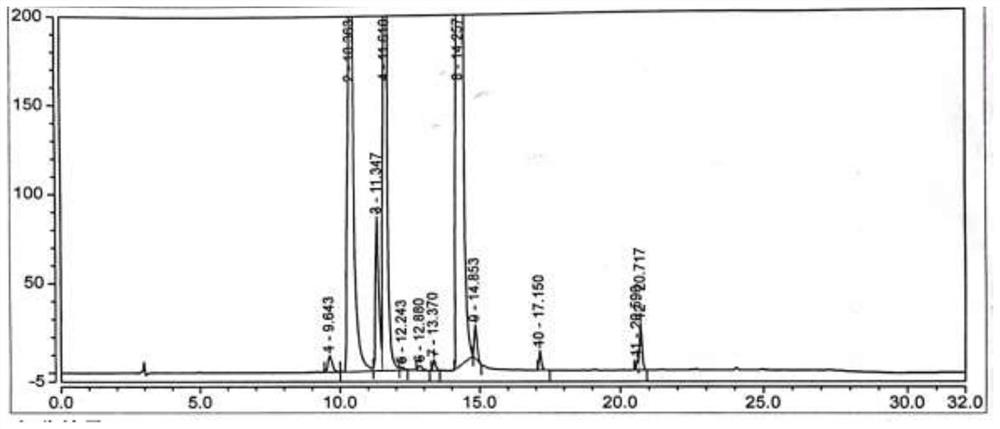
[0077] Under nitrogen protection, add the compound of formula (VI) (2.5g, 0.02mol, 1.0eq) into a 100ml reaction flask, add 50ml of acetonitrile, start stirring, heat up to reflux, add NCS (4.0g, 0.03mol, 1.5eq), AIBN (0.33g, 0.002mol, 0.1eq), reacted for 6h, TLC monitored the disappearance of the compound of formula (VI), concentrated the acetonitrile under reduced pressure, added 20ml of toluene, stirred for 30min, filtered, collected the filtrate, added dropwise 60ml of n-hexane while stirring at room temperature, After the dropwise addition was completed, the temperature was lowered to 0-10°C and stirred for 60 minutes, filtered and dried to obtain the compound of formula (Ⅴ) (2.6g), with a yield of 82.5% and an HPLC purity of 67.05%, see figure 2 .
example 3
[0078] Preparation of Example 3 Compound V
[0079] Under nitrogen protection, add the compound of formula (VI) (2.5g, 0.02mol, 1.0eq) into a 100ml reaction flask, add 50ml of acetonitrile, start stirring, heat up to reflux, add NCS (2.67g, 0.02mol, 1.0eq), AIBN (0.33g, 0.002mol, 0.1eq), reacted for 6h, TLC monitored that the compound of formula (VI) was not completely reacted.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com