Progesterone sustained-release composition and preparation method thereof
A slow-release composition and progesterone technology, which is applied in the direction of drug combination, pharmaceutical formula, drug delivery, etc., can solve the problems of large muscle irritation and poor patient compliance, and achieve simple preparation process, accurate dosage, Ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
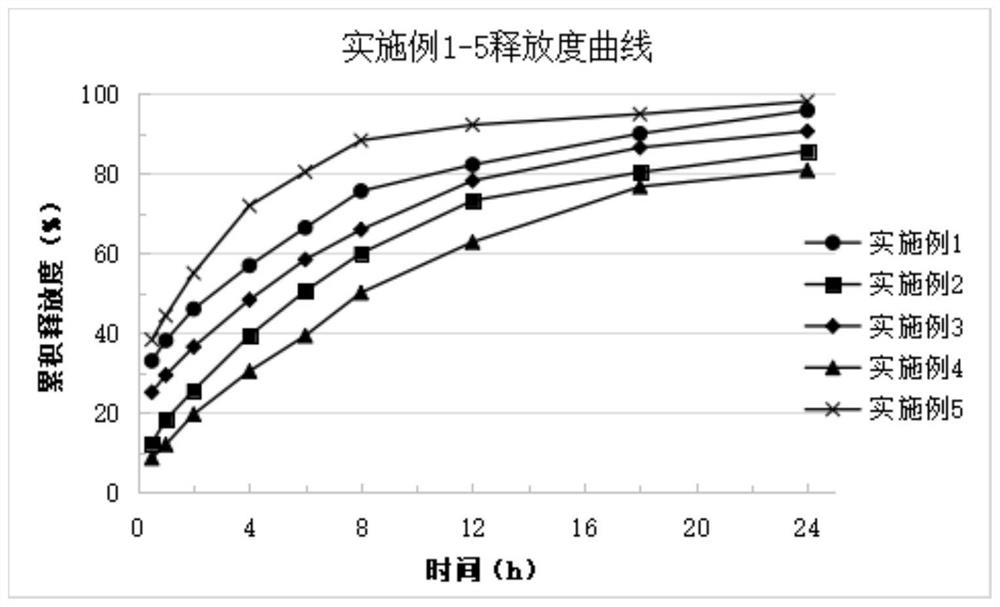
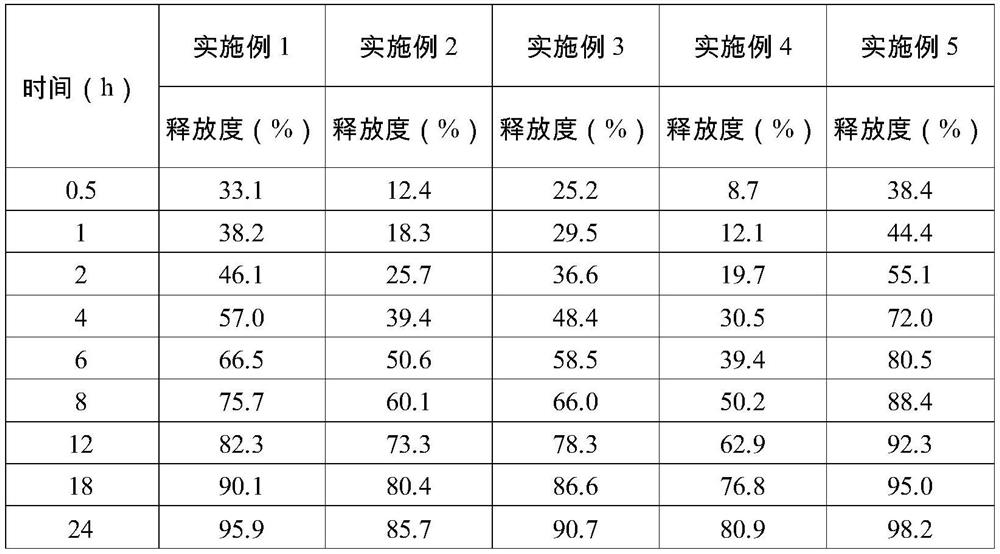
Image
Examples
Embodiment 1
[0035] Core composition 1000 pieces / g Progesterone 200 Microcrystalline cellulose 85 Granular lactose 125 Hydroxypropylketolane K15M 90 Kapom 934P 210 Citrate 100 Sodium bicarbonate 180 Magnesium stearate 10 total 1000
[0036] Preparation Process: Paltalone is pulverized first, each accessory is 30 mesh sieve, then referred to as part of the amount of progesterone, microcrystalline cellulose, granulated lactose, hydroxypropylttellulose, carbum, sodium bicarbonate 30 min was mixed in the mixer, then mixed with magnesium stearate for 5 min, and a 3.5 * 10.5 mm shaped punching piece, the sheet hardness was about 10 kg, and the range was 8 to 12 kg.
Embodiment 2
[0038] Core composition 1000 pieces / g Progesterone 200 Microcrystalline cellulose 85 Granular lactose 125 Hydroxypropylketolane K15M 210 Kapom 934P 90 Citrate 100 Sodium bicarbonate 180 Magnesium stearate 10 total 1000
[0039] Preparation Process: Paltalone is pulverized first, each accessory is 30 mesh sieve, then referred to as part of the amount of progesterone, microcrystalline cellulose, granulated lactose, hydroxypropylttellulose, carbum, sodium bicarbonate 30 min was mixed in the mixer, then mixed with magnesium stearate for 5 min, and a 3.5 * 10.5 mm shaped punching piece, the sheet hardness was about 10 kg, and the range was 8 to 12 kg.
Embodiment 3
[0041] Core composition 1000 pieces / g Progesterone 200 Microcrystalline cellulose 85 Granular lactose 125 Hydroxypropylketolane K15M 150 Kapom 934P 150 Citrate 100 Sodium bicarbonate 180 Magnesium stearate 10 total 1000
[0042] Preparation Process: Paltalone is pulverized first, each accessory is 30 mesh sieve, then referred to as part of the amount of progesterone, microcrystalline cellulose, granulated lactose, hydroxypropylttellulose, carbum, sodium bicarbonate 30 min was mixed in the mixer, then mixed with magnesium stearate for 5 min, and a 3.5 * 10.5 mm shaped punching piece, the sheet hardness was about 10 kg, and the range was 8 to 12 kg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com