Method for detecting gentamicin C1a as starting material of etimicin
A technology of gentamicin and etimicin, which is applied in the field of chemical analysis, can solve the problems of inability to correspond to impurities, poor separation of impurities, and large gaps, and achieve good specificity and rapid and accurate detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0028] The present embodiment detects the gentamycin C in the etimicin desorption solution 1a , directly detected without preprocessing.
[0029] Chromatographic conditions: packing particle size 5μm, column size 150mm×6.6mm.
[0030] In the mobile phase, acetonitrile:0.01M trifluoroacetic acid=35:65; flow rate 0.5mL / min.
[0031] The injection volume is 10 μL.
[0032] The derivatization reagent of this embodiment: Weigh 10 g of boric acid, 5 g of sodium hydroxide, add 800 mL of water to dissolve; weigh 200 mg of o-phthalaldehyde, dissolve with 10 mL of methanol, add the o-phthalaldehyde solution into the boric acid buffer solution, and dilute with water to 1L, then add 0.5mL of thioglycolic acid, mix well for later use, and prepare immediately for use.
[0033] Derivatization conditions: the flow rate of the derivatization reagent is 0.5 mL / min, and the derivatization reaction temperature is 45°C.
[0034] The detection wavelength is 330nm.
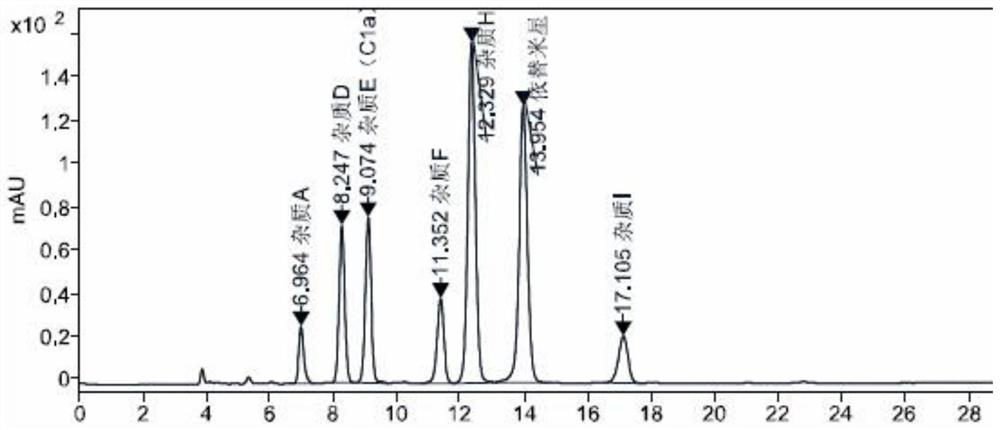
[0035] The specificity map ...
Embodiment 2)
[0038] Gentamicin C of the present embodiment 1a The detection method all the other are identical with embodiment 1, difference is:
[0039] Chromatographic conditions: packing particle size 4μm, column size 150mm×5mm.
[0040] In the mobile phase, acetonitrile:0.01M trifluoroacetic acid=30:70; flow rate 0.5mL / min.
[0041] The injection volume is 10 μL.
[0042] Derivatization conditions: the flow rate of the derivatization reagent is 0.5 mL / min, and the derivatization reaction temperature is 50°C.
[0043] The detection wavelength is 335nm.
[0044] Test result: Gentamicin C 1a The main impurities in the test substance and the main impurity were all achieved baseline separation.
Embodiment 3)
[0046] Gentamicin C of the present embodiment 1a The detection method all the other are identical with embodiment 1, difference is:
[0047] Chromatographic conditions: packing particle size 6μm, column size 250mm×6.6mm.
[0048] In the mobile phase, methanol:0.02M trifluoroacetic acid=20:80; flow rate 1.0mL / min.
[0049] The injection volume is 20 μL.
[0050] Derivatization conditions: the flow rate of the derivatization reagent is 1.0 mL / min, and the derivatization reaction temperature is 65°C.
[0051] The detection wavelength is 350nm.
[0052] Test result: Gentamicin C 1a The main impurities in the test substance and the main impurity were all achieved baseline separation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com