Fluoride photo-thermal difunctional nano material and preparation method thereof
A nanomaterial, dual-function technology, applied in the nanometer metal fluoride preparation technology and photothermal field, to achieve excellent photoluminescence properties, excellent photothermal properties, and excellent luminescence effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
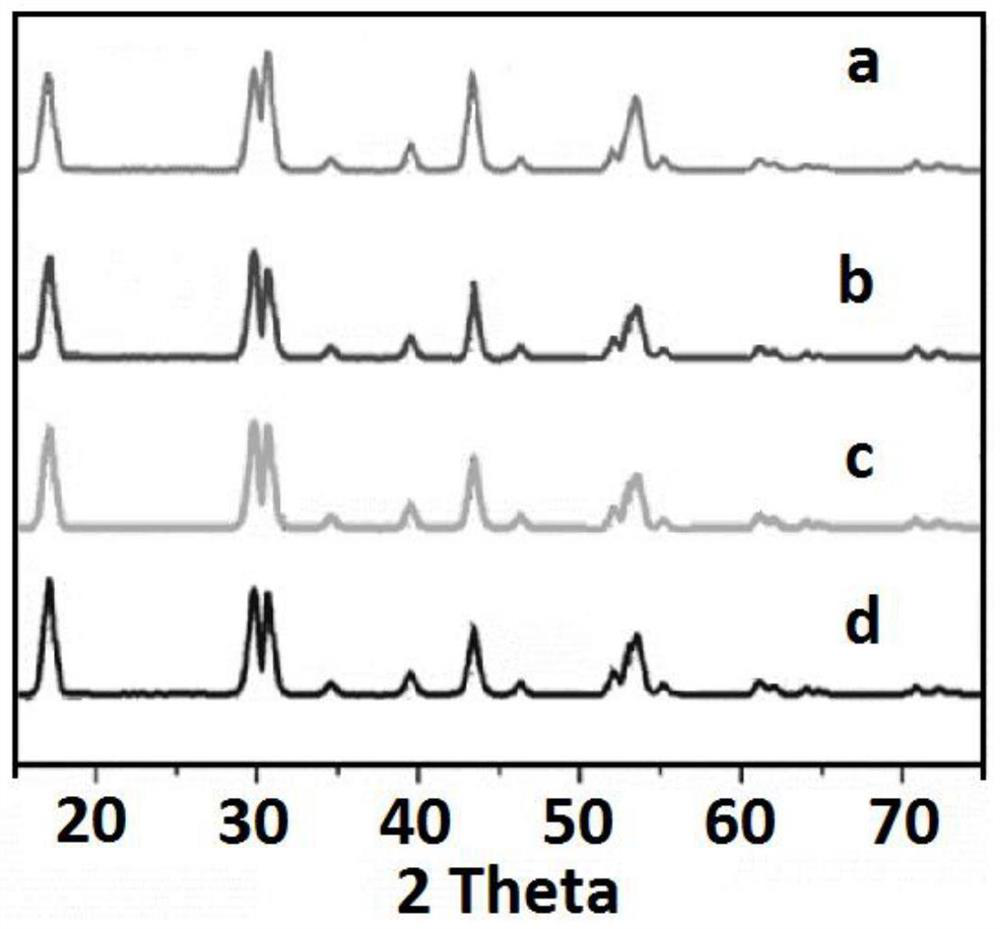
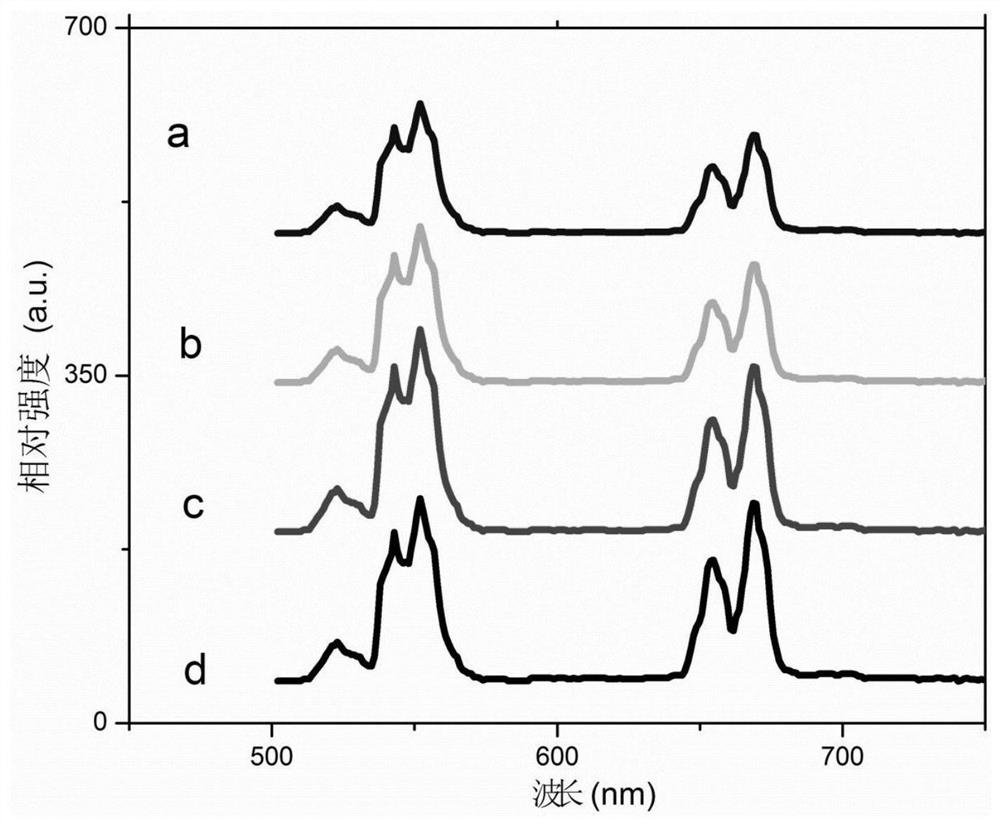
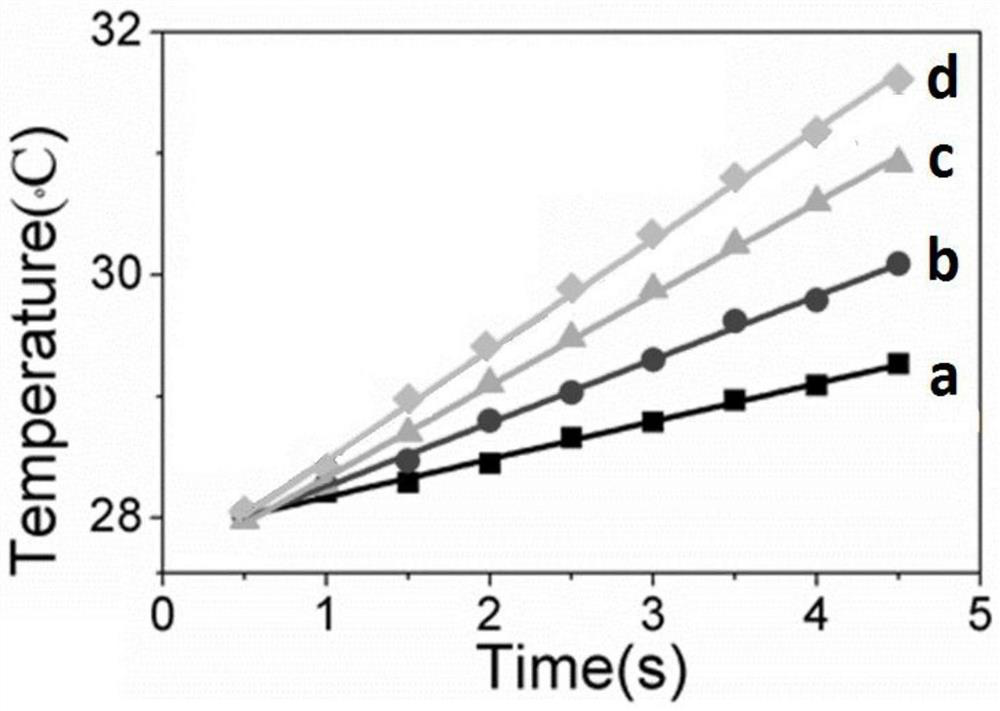
Embodiment 1
[0041] S1: Add oleic acid, octadecene and octadecylamine into a three-necked flask at a volume ratio of 50:45:5, with a total volume of 20ml; then add 0.5% of the total mass of sodium oleate, and heat and stir to form a uniform solvent;
[0042] S2: Add 1 mmol lithium chloride, 0.7 mmol lanthanum chloride, 0.17 mmol ytterbium chloride, 0.03 mmol erbium chloride, 0.1 mmol aluminum chloride and 6 mmol ammonium fluoride into S1 to obtain In a solvent, the concentration of the aqueous solution of lithium chloride, lanthanum chloride, ytterbium chloride, erbium chloride, aluminum chloride and ammonium fluoride is 1mol / L;
[0043] S3: heat the solution obtained in S2 at 160°C for 40 minutes, evaporate and remove water, and obtain an emulsion;
[0044] S4: Vacuumize the emulsion obtained in S3, then raise the temperature at a rate of 30°C / min and pass it through 3.5m 3 / min of fast-flow argon, and then reduce to 0.8m after reaching a temperature of 325°C 3 / min slow-flow argon, and...
Embodiment 2
[0050] S1: Add oleic acid, octadecene and octadecylamine into a three-necked flask at a volume ratio of 50:45:5, with a total volume of 20ml; then add 0.5% of the total mass of sodium oleate, and heat and stir to form a uniform solvent;
[0051] S2: The aqueous solution of 1 mmol lithium chloride, 0.61 mmol lanthanum chloride, 0.17 mmol ytterbium chloride, 0.03 mmol erbium chloride, 0.19 mmol aluminum chloride and 6 mmol ammonium fluoride is dropped into S1 in sequence In the obtained solvent, wherein the concentration of the aqueous solution of lithium chloride, lanthanum chloride, ytterbium chloride, erbium chloride, aluminum chloride and ammonium fluoride is 1mol / L;
[0052] S3: heat the solution obtained in S2 at 160°C for 40 minutes, evaporate and remove water, and obtain an emulsion;
[0053] S4: Vacuumize the emulsion obtained in S3, then raise the temperature at a rate of 30°C / min and pass it through 3.5m 3 / min of fast-flow argon, and then reduce to 0.8m after reachi...
Embodiment 3
[0059] S1: Add oleic acid, octadecene and octadecylamine into a three-necked flask at a volume ratio of 50:45:5, with a total volume of 20ml; then add 0.5% of the total mass of sodium oleate, and heat and stir to form a uniform solvent;
[0060] S2: The aqueous solution of 1 mmol lithium chloride, 0.52 mmol lanthanum chloride, 0.17 mmol ytterbium chloride, 0.03 mmol erbium chloride, 0.28 mmol aluminum chloride and 6 mmol ammonium fluoride is dropped into S1 in sequence In the obtained solvent, wherein the concentration of the aqueous solution of lithium chloride, lanthanum chloride, ytterbium chloride, erbium chloride, aluminum chloride and ammonium fluoride is 1mol / L;
[0061] S3: heat the solution obtained in S2 at 160°C for 40 minutes, evaporate and remove water, and obtain an emulsion;
[0062] S4: Vacuum the emulsion obtained in S3, then raise the temperature at a rate of 30°C per minute and pass it through 3.5m 3 / min of fast-flow argon, and then reduce to 0.8m after re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com