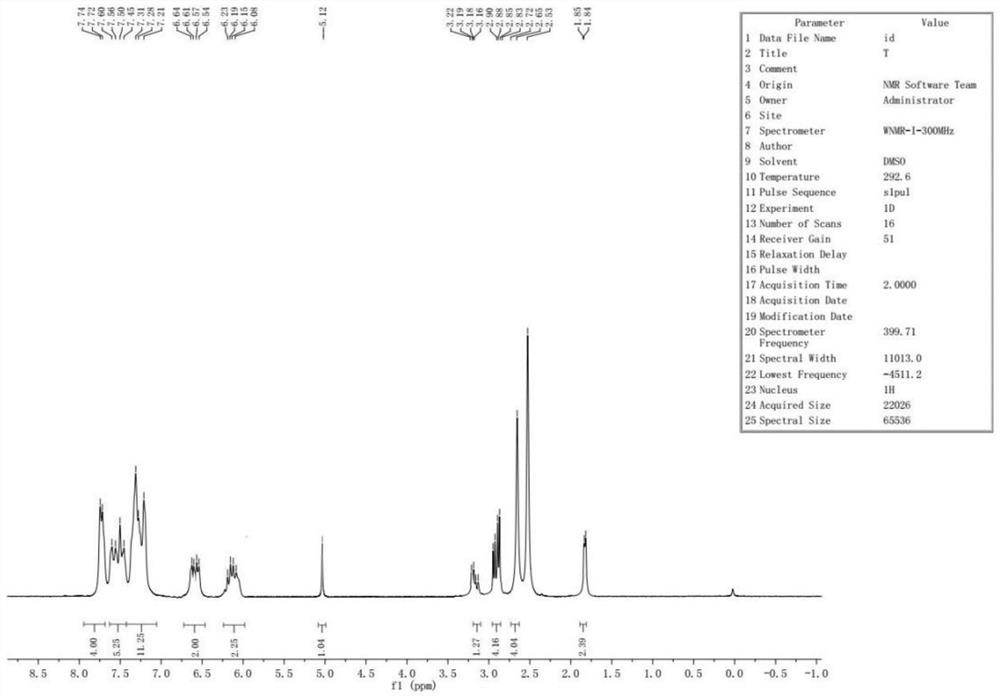
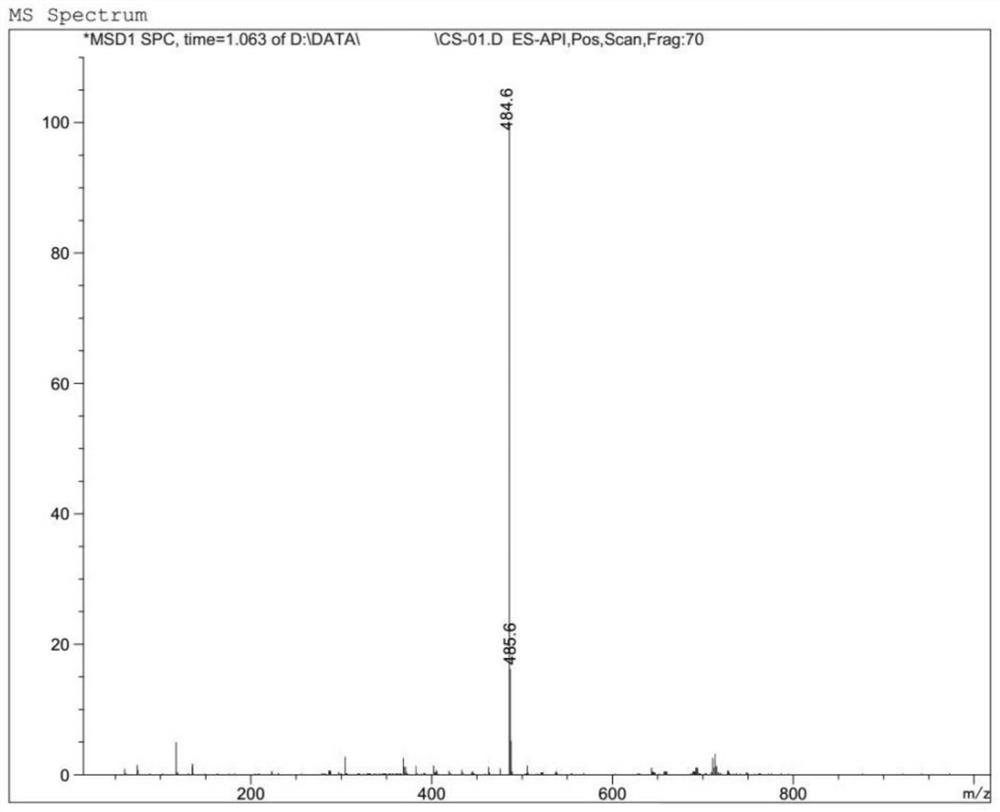
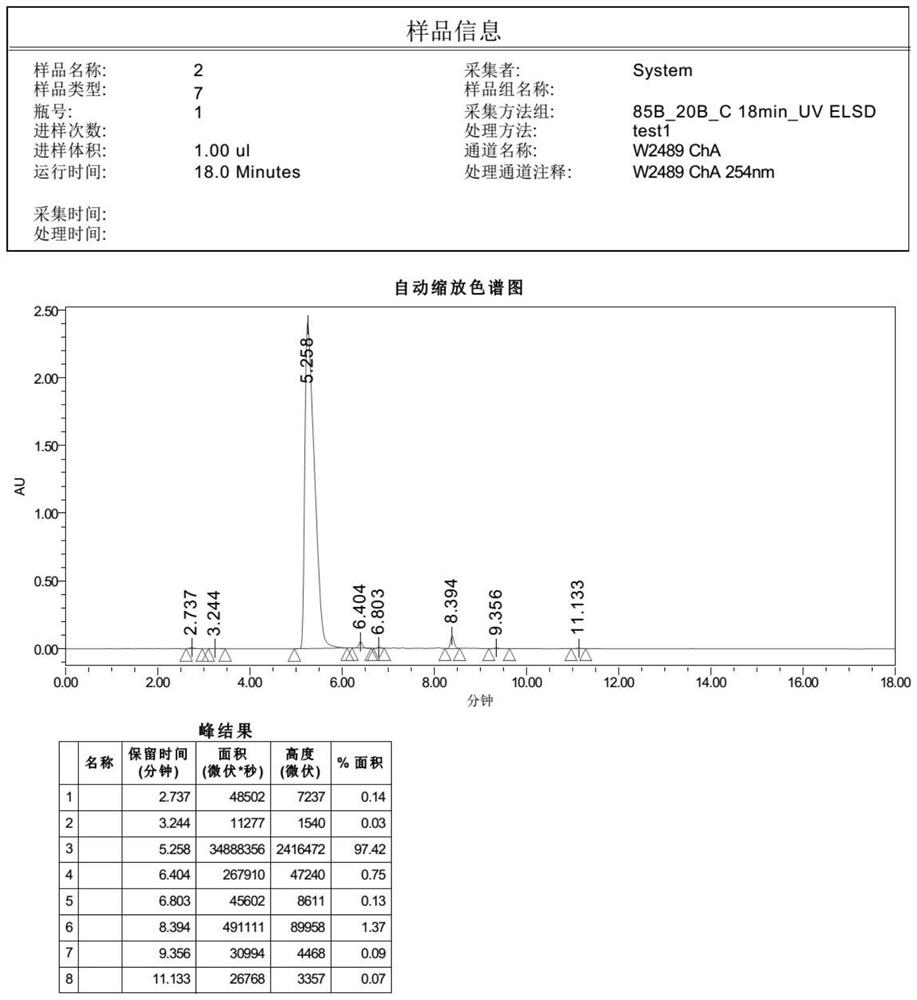
Preparation method of cinnarizine impurity and impurity
A technology of cinnarizine and impurities, which is applied to the preparation method of cinnarizine impurities and the field of impurities, and can solve the problems of less research on the preparation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Cinnarizine impurity preparation method comprises the steps:
[0043] step one:
[0044] Weigh 30g of cinnamyl alcohol, 200ml of dichloromethane, add dropwise 60g of phosphorus tribromide at 0°C, keep the temperature at 0°C for 12 hours, and the reaction is completed;
[0045] Step two:
[0046] Step 1 After the reaction, add 200ml of anhydrous tetrahydrofuran, 20g of zinc powder, and 200mg of iodine, heat to reflux, then remove the heat source, stir at room temperature for 30min, cool down to 0°C, add 6g of cinnamaldehyde dropwise, stir for 30min, and the reaction is complete;
[0047] Step three:
[0048] After the reaction in step 2, add 200ml of dichloromethane, add dropwise 8g of phosphorus tribromide at 0°C, keep the temperature at 0°C for 12 hours, and the reaction is completed;
[0049] Step four:
[0050] After the reaction in step 2, add 6.3g of compound 1, 2.5g of potassium carbonate, 50ml of 1,4-dioxane, and heat to reflux for 12 hours. After the reactio...
Embodiment 2
[0052] step one:
[0053] Weigh 30g of cinnamyl alcohol, 100ml of dichloromethane, add dropwise 50g of phosphorus tribromide at 0°C, keep the temperature at 0°C for 12 hours, and the reaction is completed;
[0054] Step two:
[0055] Step 1 After the reaction, add 100ml of anhydrous tetrahydrofuran, 25g of zinc powder, and 200mg of iodine, heat to reflux, then remove the heat source, stir at room temperature for 30min, cool down to 0°C, add 8g of cinnamaldehyde dropwise, stir for 30min, and the reaction is complete;
[0056] Step three:
[0057] Step 3 After the reaction, add 100ml of dichloromethane, add 12g of phosphorus tribromide dropwise at 0°C, keep the temperature at 0°C for 12 hours, and the reaction is completed;
[0058] Step four:
[0059]After the reaction in Step 3, add 6.8g of compound 1, 3.5g of potassium carbonate, 50ml of 1,4-dioxane, and heat to reflux for 12 hours. After the reaction is complete, filter and concentrate the filtrate under reduced pressure....
Embodiment 3
[0061] step one:
[0062] Weigh 30g of cinnamyl alcohol, 200ml of dichloromethane, add dropwise 40g of phosphorus tribromide at 0°C, keep the temperature at 0°C for 12 hours, and the reaction is completed;
[0063] Step two:
[0064] Step 1 After the reaction, add 200ml of anhydrous tetrahydrofuran, 30g of zinc powder, and 500mg of iodine, heat to reflux, then remove the heat source, stir at room temperature for 30min, cool down to 0°C, add 9g of cinnamaldehyde dropwise, stir for 30min, and the reaction is complete;
[0065] Step three:
[0066] After the reaction in step 2, add 200ml of dichloromethane, add 15g of phosphorus tribromide dropwise at 0°C, keep the temperature at 0°C for 12 hours, and the reaction is completed;
[0067] Step four:
[0068] After the reaction in Step 3, add 7.3g of compound 1, 4.5g of potassium carbonate, 50ml of 1,4-dioxane, and heat to reflux for 12 hours. After the reaction is complete, filter and concentrate the filtrate under reduced press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com