Indole compound and application thereof
A technology of indole compounds and compounds, which is applied in the field of indole compounds and their application in the preparation of lenses, can solve the problems of inconspicuous anti-blue light effects, reduced light transmittance, and high production costs, achieving significant anti-blue light, adding The effect of reducing the amount of the effect is remarkable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
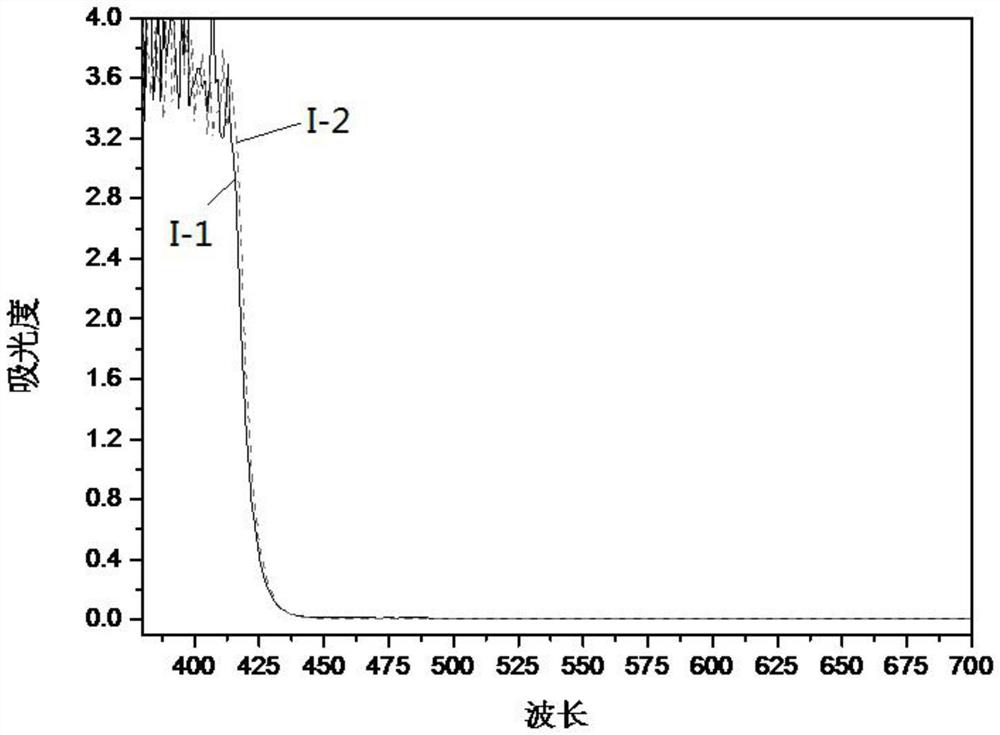
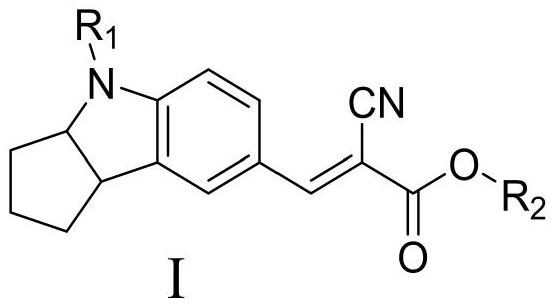
Image
Examples
Embodiment 1
[0042]
[0043] In the first step, the compound (15.9 g, 0.1 mol) shown in dry formula (II) and bromoethane (10.9 g, 0.1 mol) were dissolved in 230 mL of anhydrous tetrahydrofuran, added to a dry four-necked flask, and stirred at room temperature 2h, the reaction solution was poured into a large amount of water, extracted with dichloromethane (100mL), washed five times with water (5×100mL), the organic phases were combined, and washed with Na 2 SO 4 After drying, filtering, the organic phase was spin-dried, and separated by column chromatography, using 2 / 8 ethyl acetate / n-hexane as eluent to obtain off-white solid product (IIIa). NMR showed that the product had a structure consistent with 4-ethyl-1,2,3,3a,4,8b-hexahydrocyclopenta[b]indole. HRMS(ESI,m / z):[M+H] + calcd for (C 13 h 17N), 188.1434; found, 188.1336.
[0044]
[0045] In the second step, in a 500mL three-neck flask, drop into the compound (Ⅲa) (18.7g, 0.1mol) prepared in the first step, phosphorus oxychlo...
Embodiment 2
[0051]
[0052] In the first step, the compound (15.9 g, 0.1 mol) shown in dry formula (II) and p-bromoanisole (18.7 g, 0.1 mol) were dissolved in 230 mL of anhydrous tetrahydrofuran, and added to a dry four-necked flask, Stir at room temperature for 2h, pour the reaction solution into a large amount of water, extract with dichloromethane (100mL), wash with water five times (5×100mL), combine the organic phases, wash with Na 2 SO 4 After drying, filtering, and spin-drying the organic phase, an oily product (IIIb) was obtained for the next reaction. NMR showed that the product had a structure consistent with 4-(4-methoxyphenyl)-1,2,3,3a,4,8b-hexahydrocyclopenta[b]indole. HRMS(ESI,m / z):[M+H] + calcd for (C 18 h 19 NO), 266.1539; found, 266.1579.
[0053]
[0054] In the second step, in a 500mL three-neck flask, drop into the compound (Ⅲb) (26.5g, 0.1mol) prepared in the first step, phosphorus oxychloride (3.06g, 0.02mmol), DMF (73g, 1.0mol), at room temperature After...
Embodiment 3
[0061]
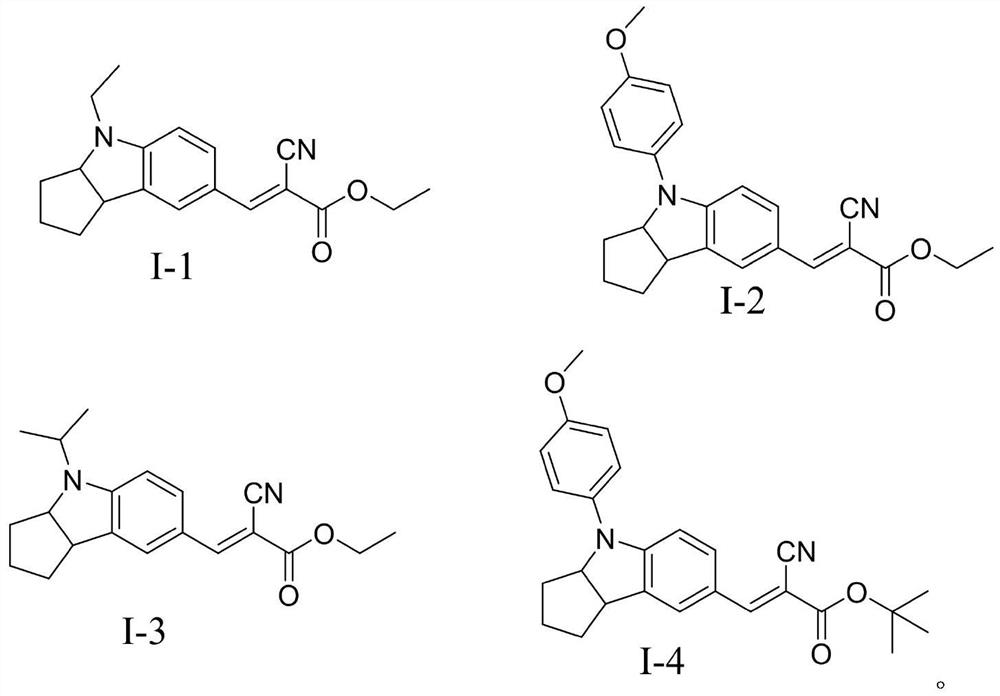
[0062] The bromoethane used in the first step reaction in Example 1 was replaced with bromoisopropane, and other steps and reagents used were the same as in Example 1 to obtain compound I-3. 1 H NMR (500MHz, CDCl 3 )δ8.23(S,1H)6.68-7.14(m,3H),3.39-4.33(q,3H),2.74-3.02(t,2H),1.12-1.93(m,15H).HRMS(ESI,m / z):[M+H] + calcd for (C 20 h 24 N 2 o 2 ), 325.1911; found, 325.1945.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com