Method for extracting and separating lithium and alkaline earth metal from salt lake brine with high sodium-lithium ratio
A salt lake brine and alkaline earth metal technology, applied in the direction of improving process efficiency, can solve the problems of extraction agent emulsification failure, long production cycle, huge salt field area, etc., to avoid small density difference between two phases, good flame retardant effect, The effect of improving safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] (1) Concentrated brine from a certain salt lake is used as the extraction water phase, and the composition of the concentrated brine is as shown in Table 1-1.
[0088] Table 1-1: The brine composition of embodiment 1
[0089]
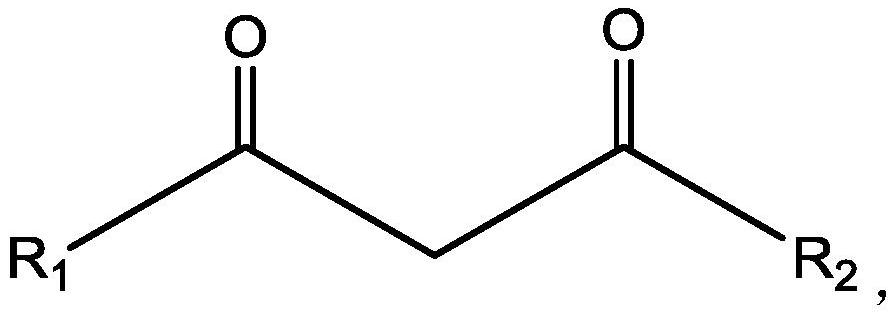
[0090] (2), preparation extraction organic phase: extraction agent is selected as benzoyl trifluoroacetone, co-extraction agent is tris (2-chloropropyl) phosphate, diluent is kerosene, and the concentration of extraction agent and co-extraction agent are respectively 0.5mol / L.
[0091] (3) Extraction: Use 4mol / L sodium hydroxide solution to saponify the extracted organic phase under the condition of 1:10, and then extract the pretreated organic phase and extracted water phase according to the volume Mixing at a ratio of 1:1 for 4-stage countercurrent extraction, the single-stage extraction time is 5min, and the phases are separated after the extraction is balanced to obtain the raffinate and the first loaded organic phase.
[0092] (4), Sodi...
Embodiment 2
[0101] (1) Concentrated brine from a certain salt lake is used as the extraction water phase, and the composition of the concentrated brine is as shown in Table 2-1.
[0102] Table 2-1: The brine composition of embodiment 2
[0103]
[0104] (2), preparation and extraction of the organic phase: the extraction agent is selected as benzoyl trifluoroacetone, the co-extraction agent is tris(1,3-dichloro-2-propyl) phosphate, the diluent is kerosene, the extraction agent and co-extraction agent The concentrations of the extractants are respectively 0.3mol / L.
[0105] (3) Extraction: Use 2mol / L potassium hydroxide solution to saponify the extracted organic phase under the condition of 1:5 ratio, and then extract the pretreated organic phase and the extracted aqueous phase according to the volume Mixing at a ratio of 1:2 for 4-stage countercurrent extraction, the single-stage extraction time is 8 minutes, and the phases are separated after the extraction is balanced to obtain the ...
Embodiment 3
[0115] (1) Concentrated brine from a certain salt lake is used as the extraction water phase, and the composition of the concentrated brine is shown in Table 3-1.
[0116] Table 3-1: The brine composition of embodiment 3
[0117]
[0118] (2), preparation and extraction of the organic phase: the extraction agent is selected as benzoyltrifluoroacetone, the co-extraction agent is tris(1-bromobutyl) phosphate, the diluent is an ionic liquid, and the concentrations of the extraction agent and the co-extraction agent are respectively It is 0.4mol / L.
[0119] (3) Extraction: Use 0.1mol / L sodium carbonate solution to saponify the extracted organic phase under the condition of 2:1 ratio, and then extract the pretreated organic phase and extracted water phase according to the volume Mixing at a ratio of 2:1 for 5-stage countercurrent extraction, the single-stage extraction time is 6 minutes, and the phases are separated after the extraction is balanced to obtain the raffinate and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com