Isorcryptolepine analogue, preparation method of isorcryptolepine analogue from lomefloxacin, and application thereof
A technology of isoflephyrine and selemenine, applied in the field of medicinal chemistry, can solve the problems of few reports of anti-tuberculosis activity, low bioavailability, poor water solubility, etc. The effect of low drug resistance and good growth inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
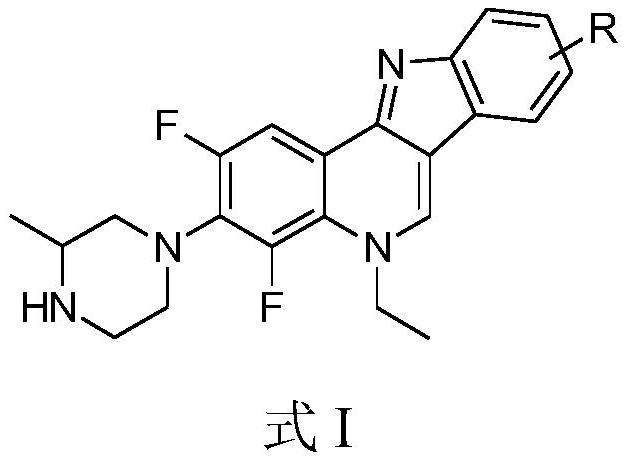
[0043] A kind of isoalbinine analogue, the general chemical structure formula is as shown in formula I:
[0044]
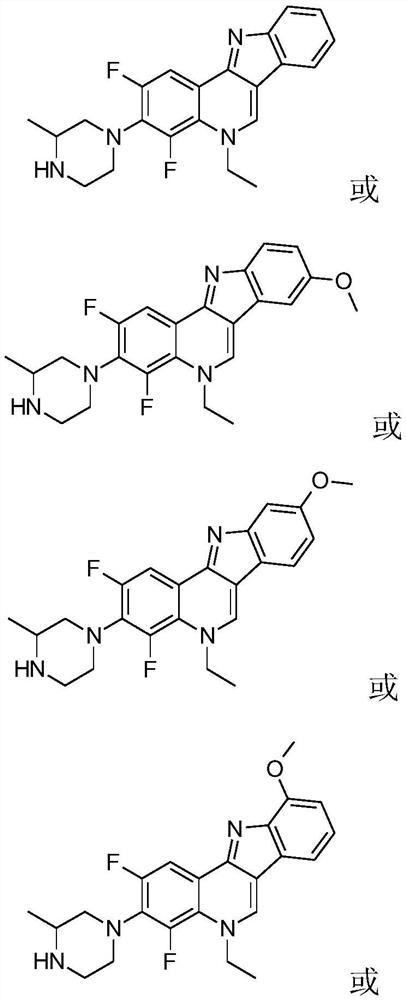
[0045] In this example, the substituent R in formula I is an H atom, then 2,4-difluoro-3-(3-methyl-piperazin-1-yl)-5-ethyl-5H-indole The chemical structural formula of [3,2-c] quinoline is:
[0046]
[0047] This example uses a preparation method from lomefloxacin to isobrainine analogues to prepare the above-mentioned isoberaline analogues (I-1), which specifically includes the following steps:
[0048] S1. Reductive decarboxylation reaction of lomefloxacin shown in formula II with potassium borohydride to prepare 2,3-dihydroquinolin-4-one shown in formula III.
[0049]
[0050] Specifically, it includes the following steps: Y1. Mix lomefloxacin with a solvent to make a suspension, slowly add potassium borohydride to the suspension in portions under normal temperature stirring, heat the mixed reactant in a water bath, stir and reflux to react to Lomefloxa...
Embodiment 2
[0058] A kind of isoberaline analogue, the difference between this embodiment and embodiment 1 is that the substituent R in the formula I is methoxyl group, then the isoberaline analogue is 2,4-difluoro-5- The chemical structural formula of ethyl-8-methoxy-3-(3-methyl-piperazin-1-yl)-5H-indolo[3,2-c]quinoline is:
[0059]
[0060] This example uses a preparation method from lomefloxacin to isobrainine analogues to prepare the above-mentioned isobrainine analogues (I-2), which specifically includes the following steps: taking 1-ethyl- 6,8-Difluoro-(3-methyl-piperazin-1-yl)-2,3-dihydro-quinolin-4(1H)-one III 1.0g (3.2mmol) ketone was dissolved in 15mL of anhydrous Add 0.62 g (4.5 mmol) of p-methoxyphenylhydrazine to ethanol, stir and react at room temperature overnight, and an obvious precipitate is formed. Concentrated hydrochloric acid (0.50 mL) was added, and the mixed reactants were refluxed for 15 h and left overnight. Collect the resulting solid by filtration, dissolv...
Embodiment 3
[0063] A kind of isoberaline analogue, the difference between this embodiment and embodiment 1 is that the substituent R in the formula I is methoxyl group, then the isoberaline analogue is 2,4-difluoro-5- The chemical structural formula of ethyl-9-methoxy-(3-methyl-piperazin-1-yl)-5H-indolo[3,2-c]quinoline is:
[0064]
[0065] This example uses a preparation method from lomefloxacin to isobrainine analogues for the preparation of the above-mentioned isobrainine analogues (I-3), which specifically includes the following steps: taking 1-ethyl- 6,8-Difluoro-(3-methyl-piperazin-1-yl)-2,3-dihydro-quinolin-4(1H)-one III 1.0g (3.2mmol) was dissolved in 15mL absolute ethanol 0.83 g (6.0 mmol) of m-methoxyphenylhydrazine was added, stirred at room temperature for 24 hours, and an obvious precipitate was formed. Concentrated hydrochloric acid (0.50 mL) was added, and the mixed reactant was refluxed for 16 h and left overnight. Collect the resulting solid by filtration, dissolve t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com