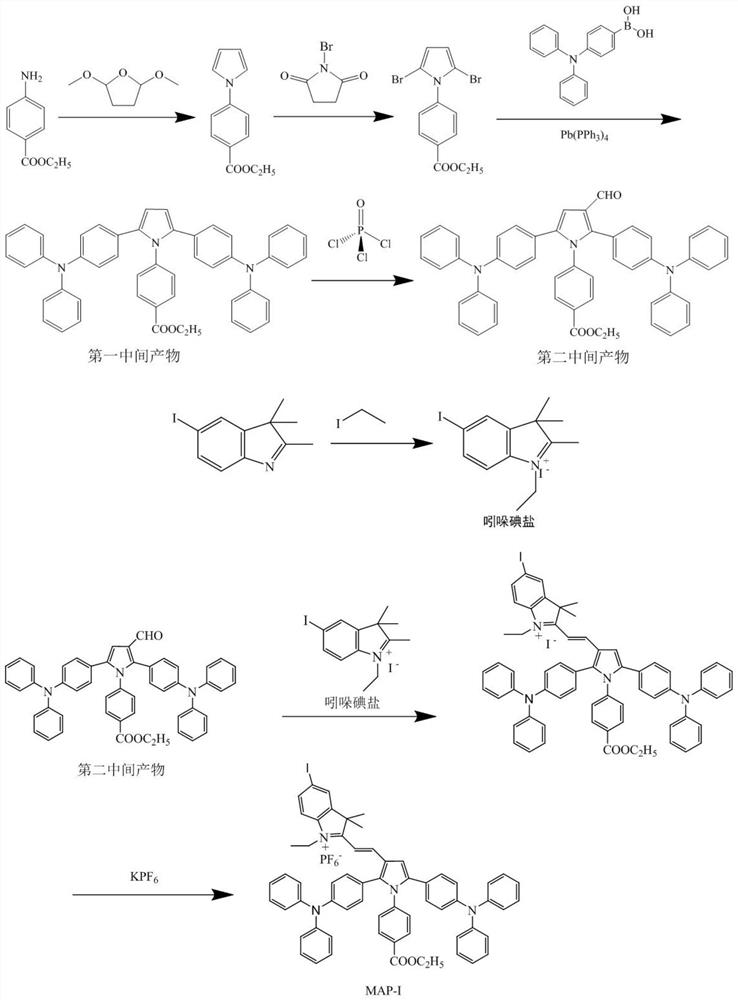
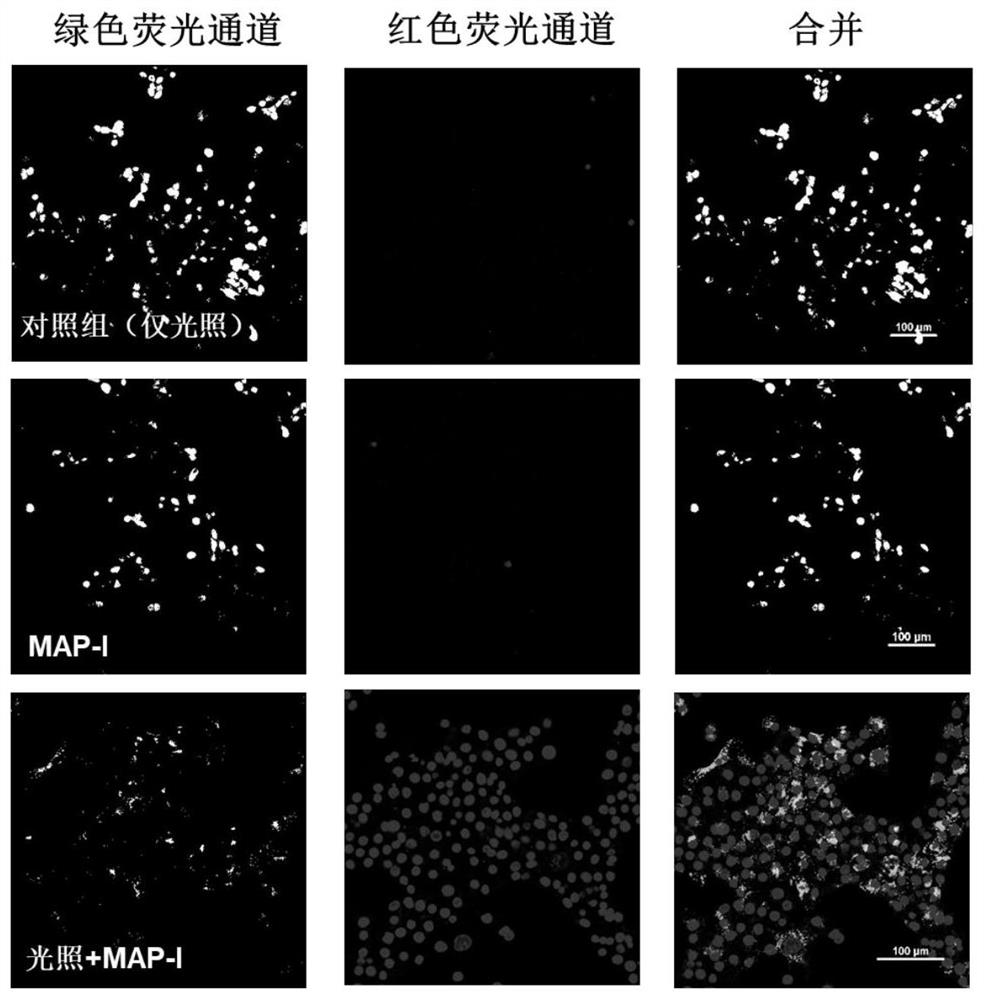
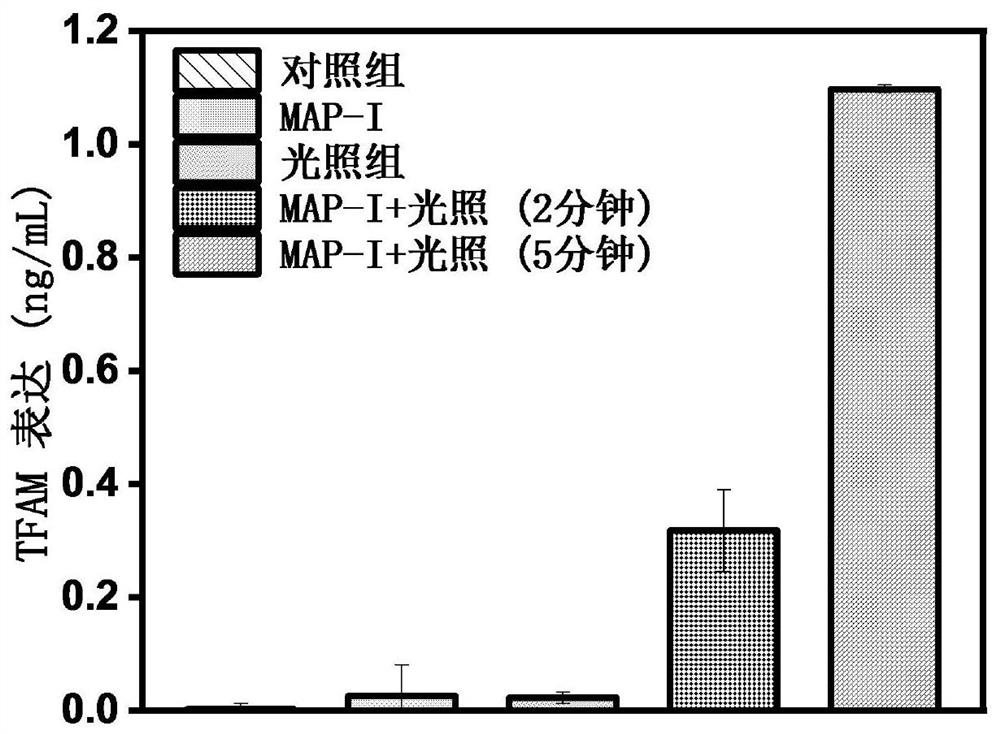
Photosensitizer for inducing death of immunogenic cells and preparation method and application thereof
A technology of cell death and immunogenicity, applied in the field of medicine, can solve the problem of less photosensitizer, achieve good biocompatibility, and achieve the effect of inducing anti-tumor immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention also provides a preparation method of the photosensitizer described in the above technical solution, comprising the following steps:
[0044] (1) Ethyl 4-aminobenzoate, 2,5-dimethoxytetrahydrofuran and the first organic solvent are mixed to react to obtain ethyl 4-(1H-pyrrol-1-yl)benzoate; 4-( 1H-pyrrol-1-yl)ethyl benzoate, N-bromosuccinimide and a second organic solvent are mixed to react to obtain 4-(2,5-dibromo-1H-pyrrol-1-yl)benzoic acid Ethyl ester; 4-(2,5-dibromo-1H-pyrrol-1-yl) ethyl benzoate, 4-triphenylamine borate, catalyst and a third organic solvent are mixed to react to obtain the first intermediate product; the The structural formula of the first intermediate product is as shown in formula 1:
[0045] Formula 1;
[0046] (2) Mix the first intermediate product, phosphorus oxychloride and the fourth organic solvent, and react to obtain the second intermediate product; the structural formula of the second intermediate product is as s...
Embodiment 1
[0110] (1) Synthesis of the first intermediate product:
[0111] Ethyl 4-aminobenzoate (1.6519 g, 10.00 mmol) and 2,5-dimethoxytetrahydrofuran (1.3216 g, 10.00 mmol) were dissolved in 25 mL of acetic acid, then refluxed for 12 h. After cooling to room temperature the solvent was removed and the residue was dissolved in dichloromethane. Wash three times with water and extract with dichloromethane. with anhydrous MgSO 4 Drying and suction filtration under reduced pressure gave the crude product, which was purified by gel chromatography using dichloromethane / petroleum ether (1 / 5, V d / V p ) mixture as eluent to give ethyl 4-(1H-pyrrol-1-yl)benzoate.
[0112] Ethyl 4-(1H-pyrrol-1-yl)benzoate (1.0970 g, 5.10 mmol) was added to 25 mL of dichloromethane. N-bromosuccinimide (1.7694g, 10.00mmol) was dissolved in 5mL of dichloromethane, and slowly dropped into the dichloromethane solution of ethyl 4-(1H-pyrrol-1-yl)benzoate under stirring, Reaction at room temperature for 12h. Th...
Embodiment 2
[0125] Synthesis of MAP-I
[0126] (1) Synthesis of the first intermediate product:
[0127] Ethyl 4-aminobenzoate (1.6519 g, 10.00 mmol) and 2,5-dimethoxytetrahydrofuran (1.0573 g, 8.00 mmol) were dissolved in 25 mL of acetic acid, then refluxed for 12 h. After cooling to room temperature the solvent was removed and the residue was dissolved in dichloromethane. Wash three times with water and extract with dichloromethane. with anhydrous MgSO 4 Drying and suction filtration under reduced pressure gave the crude product, which was purified by gel chromatography using dichloromethane / petroleum ether (1 / 5, V d / V p ) mixture as eluent to give ethyl 4-(1H-pyrrol-1-yl)benzoate.
[0128] Ethyl 4-(1H-pyrrol-1-yl)benzoate (1.0970 g, 5.10 mmol) was added to 25 mL of dichloromethane. N-bromosuccinimide (1.4155g, 8.00mmol) was dissolved in 5mL of dichloromethane, and slowly dropped into the dichloromethane solution of ethyl 4-(1H-pyrrol-1-yl)benzoate under stirring, Reaction at ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com