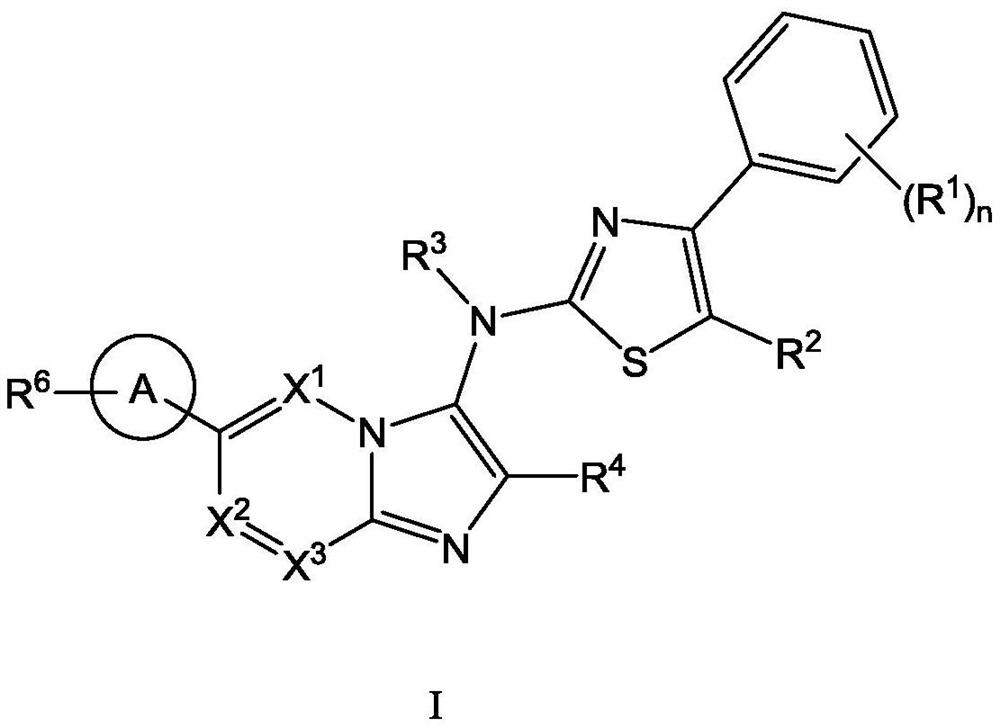
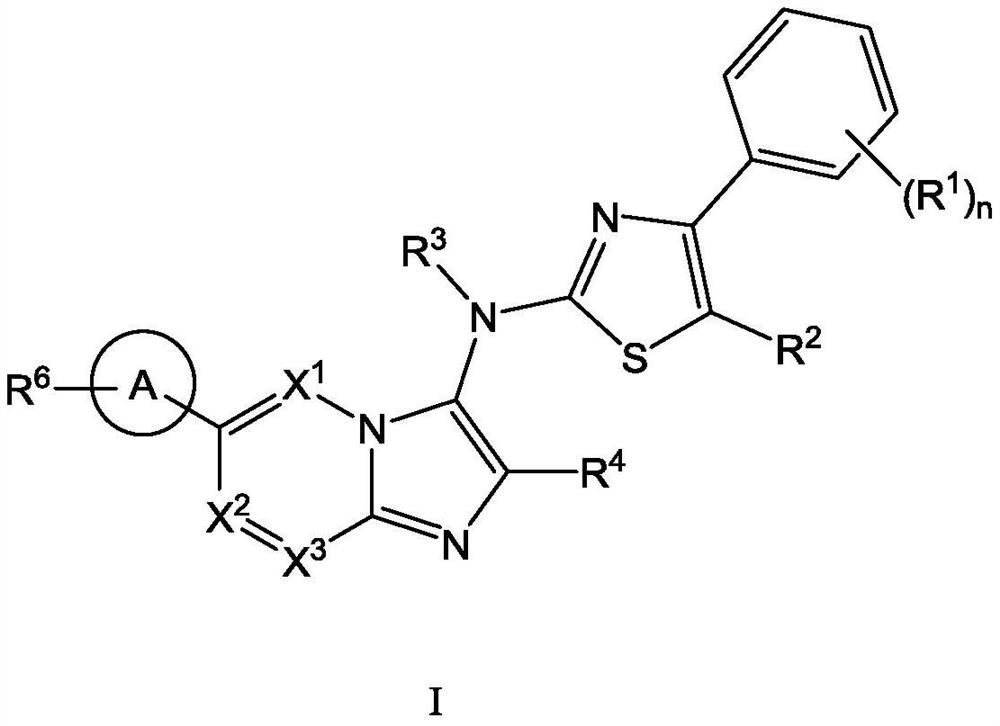
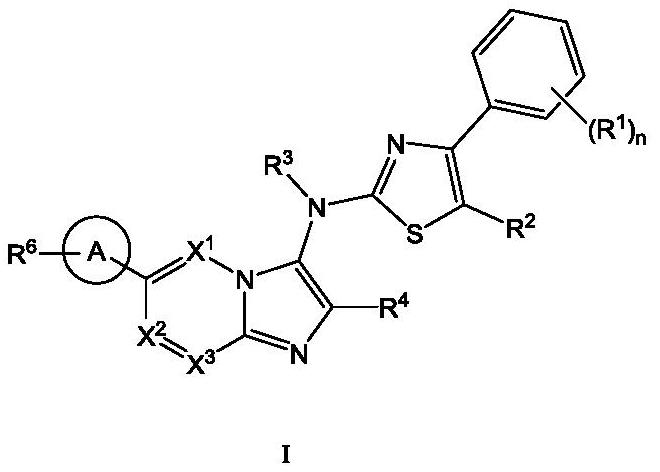
ATX inhibitor as well as preparation method and application thereof
A technology of solvate and alkyl, which is applied in the field of ATX inhibitors and its preparation, can solve the problem of low inhibitory activity and achieve excellent drug efficacy, high inhibitory activity, and high clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0111] The present invention also provides a typical synthetic method of the compound shown in the above formula I, to further describe the technical scheme of the present invention, the synthetic method comprises the following steps:
[0112] Step 1: Compound 1 reacts with the corresponding aldehyde and 1,1,3,3-tetramethylbutylisonitrile under the catalysis of magnesium chloride to generate intermediate 2;
[0113] Step 2: intermediate 2 is deprotected by heating in formic acid to obtain intermediate 3;
[0114] Step 3: Optionally, react intermediate 3 with the corresponding halogenated hydrocarbon to obtain intermediate 4 through nucleophilic substitution;
[0115] Step 4: intermediate 4 is hydrolyzed to obtain intermediate 5;
[0116] Step 5: intermediate 5 and intermediate 6 undergo a nucleophilic substitution reaction under alkaline conditions to obtain intermediate 7;
[0117] Among them, intermediate 6 is prepared by compound 11 through a two-step reaction: first, int...
Embodiment 1
[0195] 2-((2-ethyl-6-(4-(2-(3-hydroxyazetidin-1-yl)-2-oxoethyl)phenyl)imidazo[1,2-a ]pyridin-3-yl)(methyl)amino)-4-(4-fluorophenyl)thiazole-5-carbonitrile
[0196]
[0197] Step 1a: Preparation of 2-amino-4-(4-fluorophenyl)thiazole-5-carbonitrile
[0198]
[0199] The specific method of step 1a: To a solution of 3-(4-fluorophenyl)-3-oxopropionitrile (6.0 g, 36.78 mmol) in ethanol (72 mL) was added pyridine (2.97 mL, 36.78 mmol) at room temperature. The mixture was stirred at 70°C for 15 minutes and then cooled to room temperature. A solution of thiourea (5.61 g, 73.56 mmol) and iodine (9.33 g, 6.78 mmol) in EtOH (36 mL) was then added dropwise slowly. After stirring at room temperature for one hour, 1M Na 2 S 2 o 3 (36 mL) to quench the reaction. After filtration, the filter cake was washed with water and dried to give 2-amino-4-(4-fluorophenyl)thiazole-5-carbonitrile (4.2 g) as a white solid.
[0200] Step 1b: Preparation of 2-chloro-4-(4-fluorophenyl)thiazole-5-...
Embodiment 2
[0228] 2-((2-ethyl-6-(6-(2-(3-hydroxyazetidin-1-yl)-2-oxoethyl)pyridin-3-yl)imidazo[1, 2-a]pyridin-3-yl)(methyl)amino)-4-(4-fluorophenyl)thiazole-5-carbonitrile
[0229]
[0230] Step 2a: Preparation of ethyl 2-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl)acetate
[0231]
[0232] The specific method of step 2a: under nitrogen protection, add 2-(5-bromopyridin-2-yl) ethyl acetate (200mg, 0.82mmol), pinacol diborate (229mg, 0.90mmol), potassium acetate (120mg , 1.23 mmol) in 1,4-dioxane (2 mL) was added to [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (58 mg, 0.08 mmol). The mixture was heated to 85°C and stirred for 2 hours, diluted with water (15 mL), and extracted with ethyl acetate (20 mL×2). The combined organic phases were washed with saturated brine (15 mL), dried over anhydrous sodium sulfate, filtered, and concentrated to give brown oil 2-(5-(4,4,5,5-tetramethyl-1,3,2 -Dioxaborin-2-yl)pyridin-2-yl)ethyl acetate (171 mg).
[0233] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com