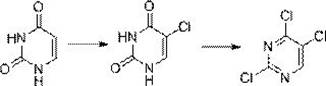
Method for preparing 2, 4, 5-trichloropyrimidine
A technology of trichloropyrimidine and trichlorouracil, applied in the field of pesticides, can solve the problems of high toxicity, harm to the health of synthesis personnel, and complicated synthesis process, and achieves low production cost, great social and economic benefits, and reasonable process conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] step 1:
[0041] Add 240g dilute sulfuric acid (concentration 9%) to a 500ml four-neck bottle, add 20g uracil, and stir for 20-30min while cooling;
[0042] At a temperature of 10-15°C, add 110g of sodium hypochlorite solution and react until the basic reaction of uracil is detected by HPLC (the content is not more than 1%);
[0043] Heated to reflux for 1-2h, then cooled to 20-30°C, filtered, washed with water, and dried to obtain 24.3g of white powder with a yield of 92.8% and a purity of over 99.5% by HPLC.
[0044] Step 2:
[0045] Add 110g tetrahydrofuran, 48g 5-chlorouracil and 0.6g DMF in the reaction flask, stir to dissolve, then add dropwise the chloroform solution of bis(trichloromethyl)carbonate, wherein the chloroform of bis(trichloromethyl)carbonate Solution is that 79g bis(trichloromethyl)carbonate is dissolved in 150g tetrahydrofuran;
[0046] After completion, heat up to 63-65°C, react until HPLC detects that the basic reaction of uracil i...
Embodiment 2
[0050]
[0051] step 1:
[0052] Add 270L of water to a 500L enamel kettle, add 26kg of 98% sulfuric acid dropwise under ice-salt cooling, cool down to 30-35°C after dropping, and add 30kg of uracil;
[0053] Cool to 15-20°C, slowly add 161 kg of sodium hypochlorite solution under stirring conditions, and stir the reaction until the basic reaction of uracil detected by HPLC is complete (the content is not more than 1%);
[0054]Raise the temperature at 95-100°C to react, after reacting for 3-4 hours, cool to 20-25°C, centrifuge, and dry to obtain 37.4kg of white powder with a yield of 95.3% and a purity of 99.1% by HPLC.
[0055] Step 2:
[0056] With 230kg chloroform, 100kg 5-chlorouracil, 1.1 kg DMF is joined in the 500 L reactor, stirring and dissolving, then add the chloroform solution (163kg bis(trichloromethyl)carbonate) of bis(trichloromethyl)carbonate dissolved in 200kg chloroform).
[0057] After the addition is complete, raise the temperature to 60-65°C and rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com