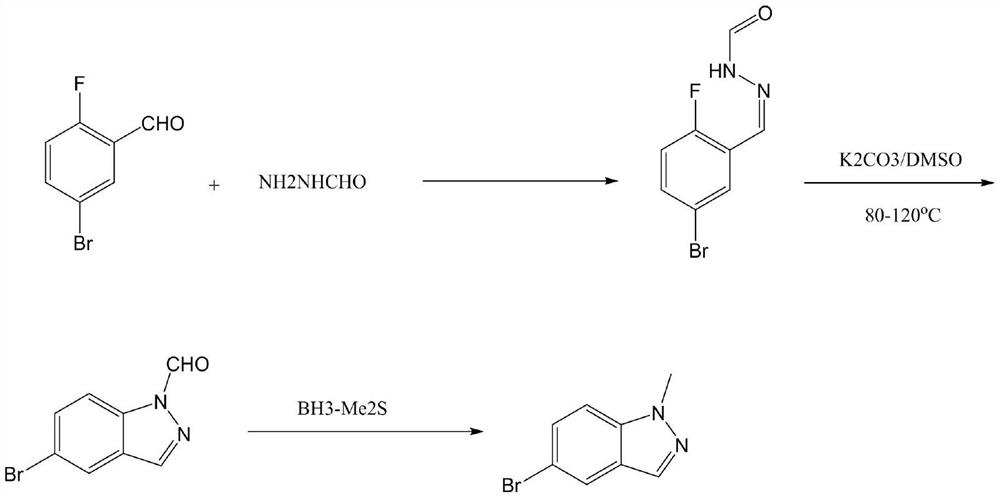
Preparation method of 5-bromo-1-methylindazole
A technology of methyl indazole and bromobenzaldehyde, applied in the direction of organic chemistry and the like, can solve problems such as low efficiency, and achieve the effects of high product purity, simple process operation and short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] Preparation of formylhydrazine
[0022] In a three-neck flask, ethyl formate (74.1 g, 1.0 mol) and 80% hydrazine hydrate (75.0 g, 1.2 mol) were added to ethanol (250 mL) respectively, and the temperature was raised to reflux for 5 hours. Excess hydrazine hydrate and ethanol solvent were distilled off under reduced pressure to obtain a light yellow oil, which solidified after cooling, and was slurried with a mixed solvent of ethanol and heptane. After drying, 55.4 g of formylhydrazine was obtained, a white crystalline solid with a melting point of 53-55°C.
Embodiment 1
[0024] In the three-necked flask, add 2-fluoro-5-bromobenzaldehyde (20.3g, 0.10mol) and 120mL absolute ethanol, and dissolve completely under stirring. Then 1.2 mL of acetic acid was added, and after cooling down to 0°C, formylhydrazine (6.0 g, 0.10 mol) was added in batches, and the addition was completed in about 20 minutes. Then naturally rise to room temperature and stir for 2 hours, and TLC detects that the reaction is complete. The reaction was directly evaporated to dryness under reduced pressure, and toluene was taken with water once to obtain a viscous solid. Add 150mL DMSO and anhydrous potassium carbonate (27.6g, 0.20mol), stir well, and heat up to 90-100°C to react overnight. TLC detected that the reaction was complete, cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure to no liquid, added 100 mL of dichloromethane and 55 mL of saturated ammonium chloride, stirred until the system was dissolved and separated, and the aque...
Embodiment 2
[0027] In the three-necked flask, add 2-fluoro-5-bromobenzaldehyde (20.3g, 0.10mol) and 120mL absolute ethanol, and dissolve completely under stirring. Then 1.2 mL of acetic acid was added, and after cooling down to 0°C, formylhydrazine (6.0 g, 0.10 mol) was added in batches, and the addition was completed in about 20 minutes. Then naturally rise to room temperature and stir for 2 hours, and TLC detects that the reaction is complete. The reaction was directly evaporated to dryness under reduced pressure, and toluene was taken with water once to obtain a viscous solid. Add 110mL of dioxane, add sodium hydride (0.16mol) in batches, stir for 5 minutes after each addition, and then add the next batch Second-rate. After all the addition was completed, the temperature was raised to 80-85°C to react overnight. TLC detected that the reaction was complete, cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure to no liquid, added 100 mL of dich...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com