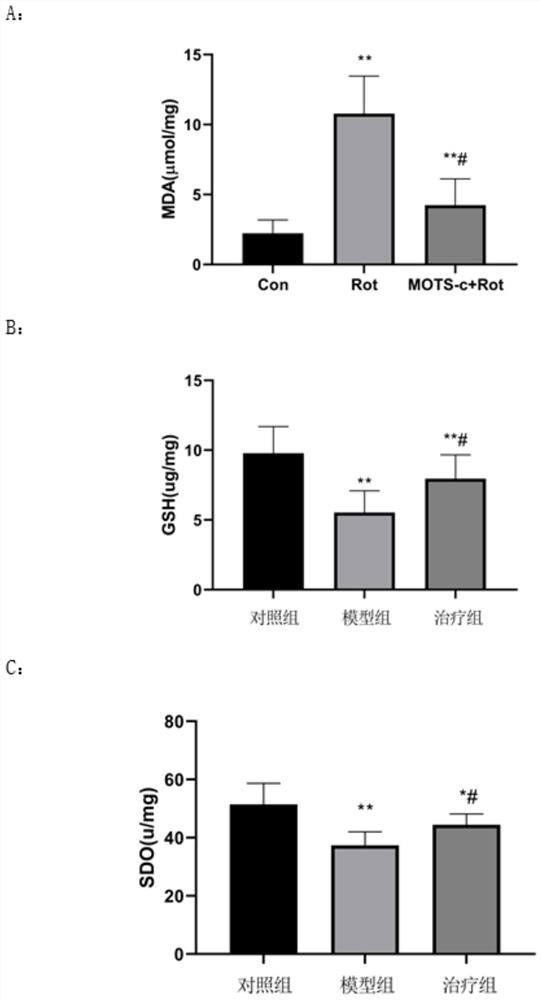
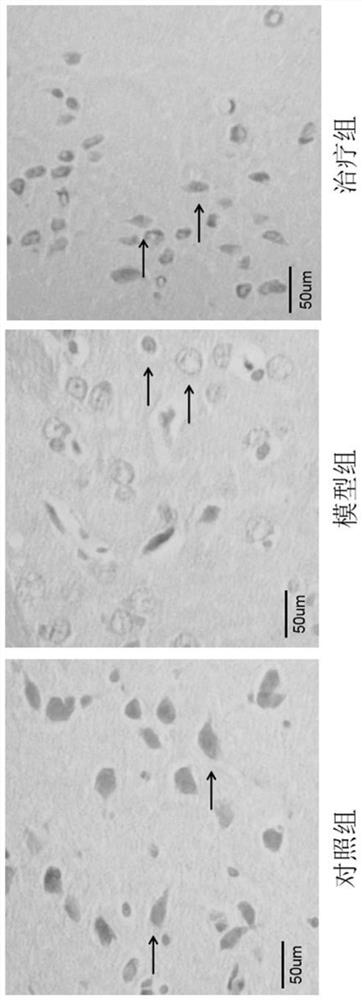
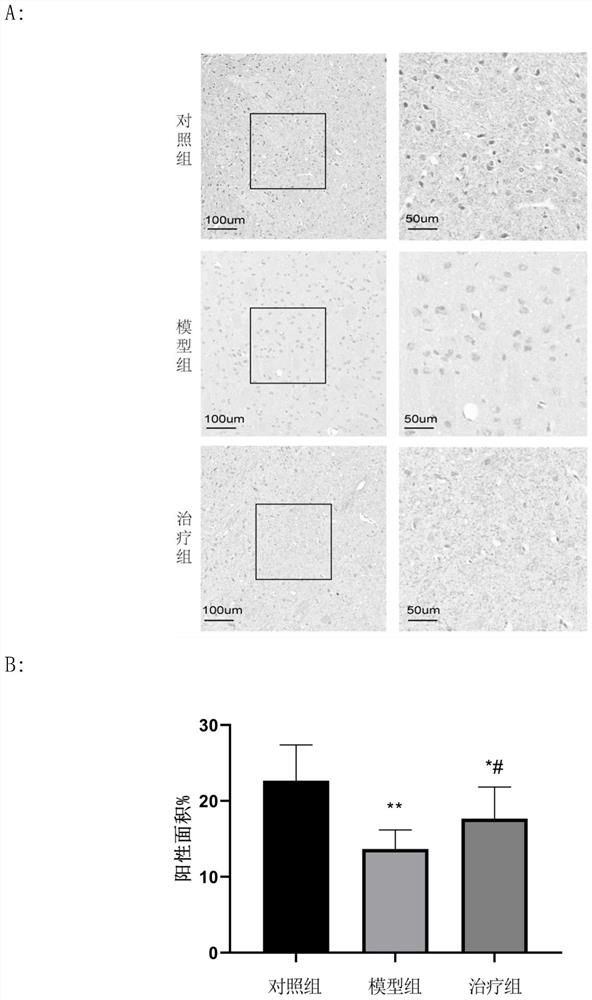
Application of peptide MOTS-c to preparation of medicine for treating Parkinson's disease
A technology of medicine, mots-c, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 animal treatment
[0034] Peptide MOTS-c was synthesized by Wuhan Baiyixin Biotechnology Co., Ltd. with a purity of >95%. The amino acid sequence is shown in SEQ.ID.NO.1:
[0035] Met Arg Trp Gln Glu Met Gly Tyr Ile Phe Tyr Pro Arg Lys Leu Arg.
[0036] 44 male SD rats, weighing between 220 and 270 g, were kept in an animal room with an indoor temperature of 23±2°C, and the rats were provided with free drinking water and food during the period. Rats were randomly divided into 3 groups: control group, model group and treatment group. Model group (gradient dose subcutaneous injection): the injection dose in the first stage is 2 mg / kg, for a total of 3 days. In the second phase, the injection dose was 1 mg / kg, and the drug was administered for 7 days. In the third stage, the injection dose is 0.5mg / kg, administered for 20 days in total. A total of 30 days of administration were used to establish a rotenone-induced rat Parkinson's animal model. Treatment g...
Embodiment 2
[0037] Embodiment 2 implements each group of rat neuroethology experimental detection
[0038] 1) Open field test: The open field test is a method to evaluate the autonomous behavior, exploration behavior and tension of experimental animals in a new environment, so as to evaluate whether the animal's activity is normal and uniform. After adjusting the software and system parameters, put the rat in the open field reaction box and let it move freely for 10 minutes, and the system will record all the activity records of the rat. After the experiment is over, the software will record and analyze parameters such as the distance of movement, the trajectory of movement, and the number of standing times of hind limbs (before each experiment, the reaction box needs to be wiped with alcohol to eliminate the influence of odor on the animals).
[0039] 2) Rotating Rod Fatigue Test: By measuring the time the animal stays on the roller, it is used to evaluate the animal's balance, motor coo...
Embodiment 3
[0040] Embodiment 3 The content of rat striatum dopamine (high resolution liquid mass spectrometry method)
[0041] The striatum of the rat was weighed and the weight was recorded, 1 mL of methanol (containing 0.1% formic acid) was precisely added, vortexed for 1 min, and homogenized for 3 min. Centrifuge at 14,000 rpm for 10 min at high speed, and take the supernatant for analysis. Chromatography Waters T3 (150 x 2.1 mm, 3 μm). The flow rate is 0.3mL / min, the aqueous phase is 0.1% formic acid aqueous solution, the organic phase is 0.1% formic acid acetonitrile, the needle washing solution is methanol, and the temperature of the column oven is 35°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com