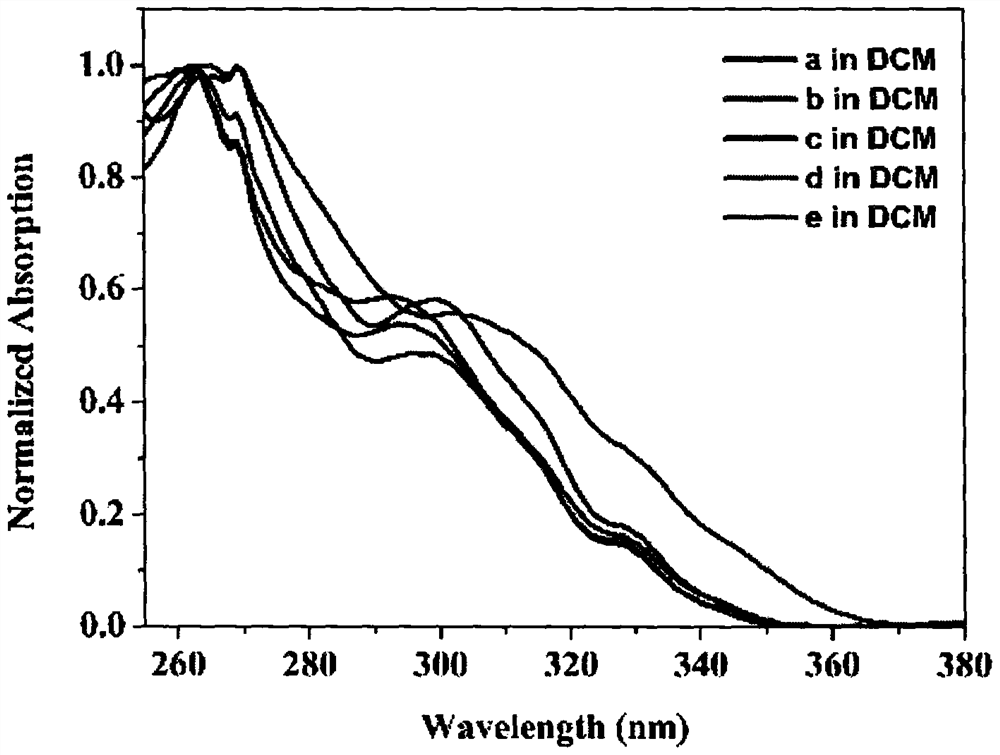
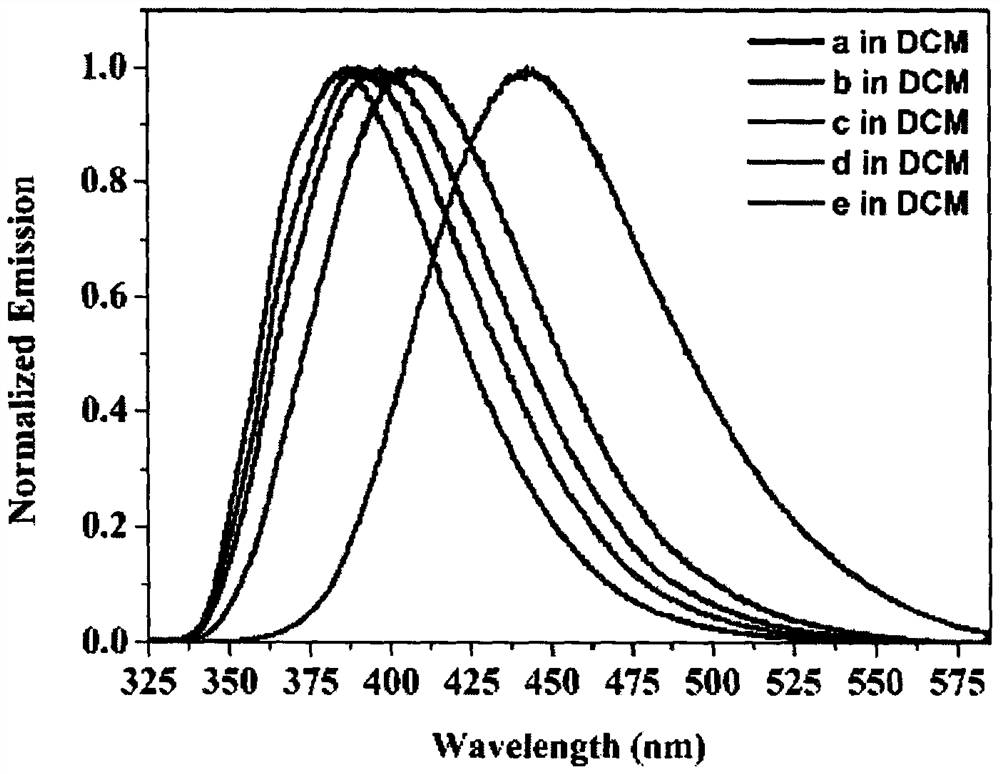
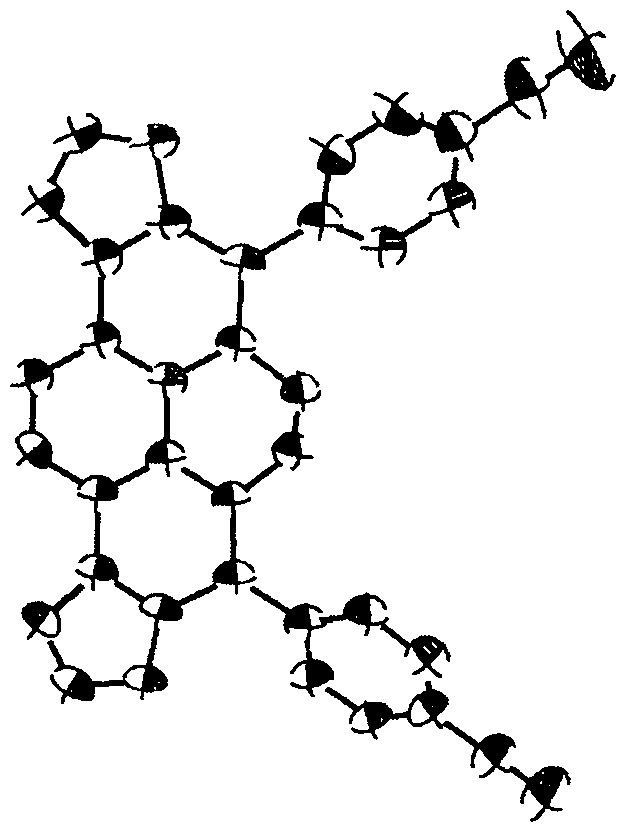
Furan fused boron aza dihydropyrene and synthesis method thereof
A technology for furan-fused boron nitrogen and heterodihydropyrene, which is applied in the field of furan-fused borazadihydropyrene and its synthesis, and can solve the problems that the potential application characteristics of luminescent materials have not been widely developed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0042] Embodiment: the synthesis of compound 1,2,3 and 4a-4e;
[0043] 1) Synthesis of Compound 1: Add 3,6-dibromobenzene-1,2-diamine (265.9mg, 1.0mmol, 1.0equiv), glyoxal (87.06mg, 1.5mmol, 1.5equiv) into a 100ml flask, Triethylamine (151.79mg, 1.5mmol, 1.5equiv) was flushed three times with argon, added an argon balloon, and then added 20mL of ethanol with a syringe, and reacted at room temperature for 16h. After the reaction was complete, dichloromethane was dissolved and washed with saturated aqueous sodium bicarbonate solution. The organic layer was collected and dried with anhydrous sodium sulfate. After filtration, the solvent was spin-dried. The product was purified by silica gel column chromatography, and eluted with ethyl acetate and petroleum ether to obtain compound 1 as a white solid.
[0044] 2) Synthesis of compound 2: add compound 1 (287.94mg, 1.0mmol, 1.0equiv) and sodium borohydride (378.25mg, 10.0mmol, 10.0equiv) in a 100ml flask, flush with argon three tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com