Injection filler containing deproteinized bone and preparation method thereof
A filler and protein bone technology, which is applied in the fields of medical cosmetology and plastic surgery, can solve the problems affecting the effectiveness of fillers, uneven product components, and inability to synergize, etc., to achieve prolonged retention time, excellent hydrophilic properties and biocompatibility Sexuality, the effect of promoting growth and differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0050] 1) Preparation of gel phase
[0051] Get the cross-linking agent BDDE and dissolve it in 1M NaOH solution according to the final concentration of 2wt%, and then add sodium hyaluronate (final concentration 15wt%) with a molecular weight of 120w-140w and recombinant collagen (final concentration) with a molecular weight of about 60K in the solution Concentration 10wt%), stir evenly, cross-link at 50°C for 4 hours. Cut the cross-linked gel into small pieces and wash it with pure water to remove reagent residues, then dilute to the final concentration of sodium hyaluronate at 20 mg / mL, the final concentration of lidocaine hydrochloride is 2mg / mL; make the final required gel (as the gel phase of the injection filler) by sieving or homogenizing.
[0052] 2) Preparation of bone pellets
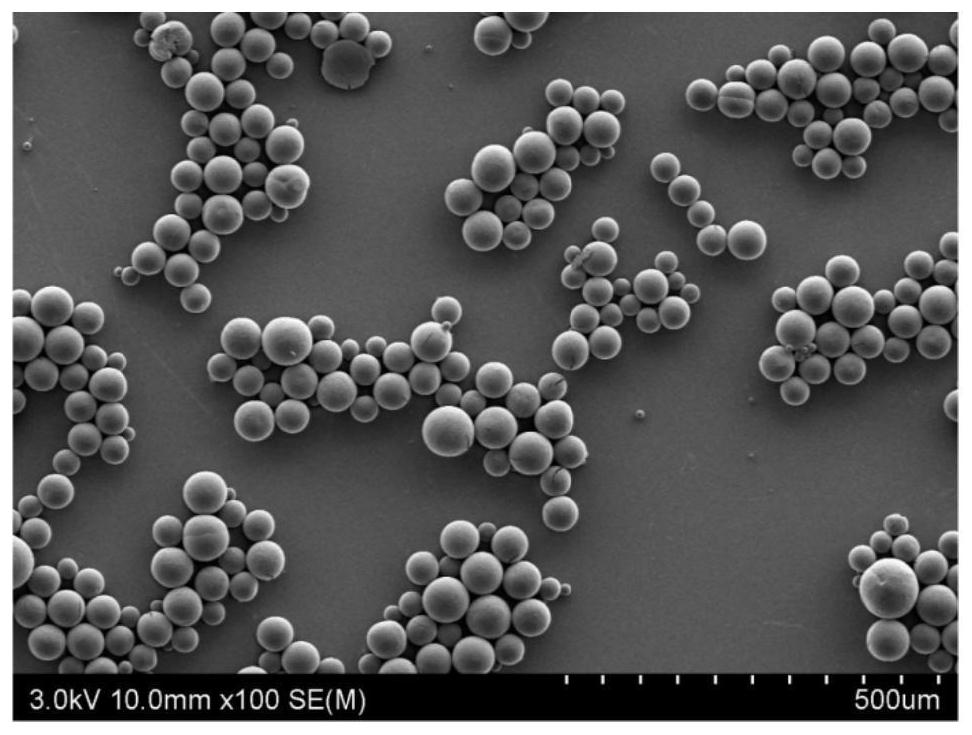
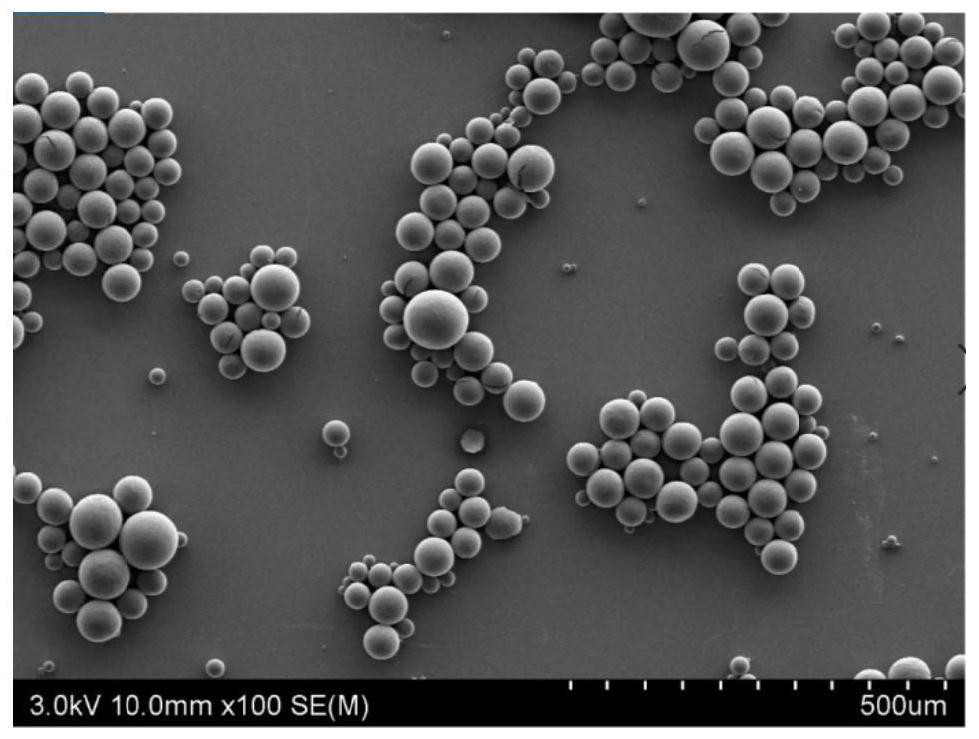
[0053] Animal (such as cattle, pigs) bone is cut into pieces, washed with pure water to no bone marrow and blood, crushed into particles with a particle diameter of 2-6mm, and then mixed wi...
example 2
[0063] 1) Preparation of gel phase
[0064] Take the cross-linking agent dimethyl sulfoxide and dissolve it in 0.25M KOH solution according to the final concentration of 4.5wt%, and then add sodium hyaluronate with a molecular weight of 220w-240w (final concentration 8wt%) and a molecular weight of about 90K to the solution. The recombinant collagen (final concentration 16wt%) was stirred evenly, and cross-linked at 40° C. for 16 hours. Cut the cross-linked gel into small pieces and wash it with pure water to remove reagent residues, then dilute to the final concentration of sodium hyaluronate at 10 mg / mL, the final concentration of benzocaine is 4mg / mL; make the final required gel (as the gel phase of the injection filler) by sieving or homogenizing.
[0065] 2) Preparation of deproteinized bone particles
[0066] Animal (such as cattle, pigs) bone is cut into pieces, washed with pure water until there is no bone marrow and blood, crushed into particles with a particle siz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com