Enzymatic preparation method of glucosamine
A glucosamine and catalytic preparation technology, applied in the field of glucosamine catalyzed by biological enzymes in vitro, enzymatic preparation of glucosamine, can solve the problems of difficulty in controlling the genetic stability of engineering bacteria and high difficulty in transforming microbial metabolic pathways, and achieve production costs Low cost, cheap raw materials, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, construction of engineered expression strain containing glucose isomerase gene and transaminase gene
[0039] According to Bacillus coagulans ( B. coagulans) of glucose isomerase, also known as xylose isomerase gene nucleotide sequence (GenBank number KYC85466) and Bacillus subtilis ( B. subtilis ) transaminase gene nucleotide sequence (GenBank number QHF59041) was synthesized separately, connected with pColdII plasmid through enzyme digestion and ligation reaction, transformed into competent cells of Escherichia coli DH5α strain, and coated with ampicillin-containing antibiotic (30μg / ml) After culturing at 37°C for 12 hours, positive transformants were picked, identified and sequenced. Inoculate the verified positive single clone into 5 mL of LB liquid medium containing 30 μg / mL ampicillin antibiotic, culture overnight at 37°C, extract two recombinant plasmids, and transform them into expression hosts E. coli In BL21(DE3), a recombinant strain was ob...
Embodiment 2
[0040] Embodiment 2, the recombinant expression preparation of glucose isomerase and transaminase
[0041] 1) Seed culture: the recombinant strain preserved on the slant E. coli BL21(DE3) / pColdII-GI and recombinant strains E. coli BL21(DE3) / pColdII-AT were respectively inoculated into LB liquid medium containing 30 μg / ml ampicillin, and cultured at 37°C for 8-10 hours to obtain seed liquid;
[0042] 2) Fermentation culture: Inoculate the seed solution with an inoculum size of 1% volume concentration into LB liquid medium containing 30 μg / ml ampicillin, and culture it at 37°C until OD 600 The value is 0.5, then transferred to 15°C for culture and added isopropyl-β-D-galactoside (IPTG) with a final concentration of 0.1mM, the rotation speed was 160 rpm, and the expression was induced for 24h. Amide gel electrophoresis (SDS-PAGE) analysis of the total protein of the whole bacteria showed that the genetically engineered bacteria had obvious recombinantly expressed protein ba...
Embodiment 3
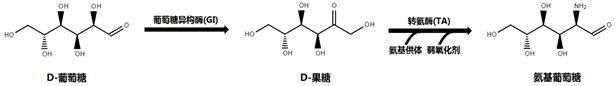
[0043] Embodiment 3, an enzyme-catalyzed preparation method of glucosamine,
[0044] The method comprises: using the glucose isomerase prepared in Example 2 to catalyze D-glucose into D-fructose; For glucosamine.
[0045] The amino donor compound is selected from D-alanine, isopropylamine, tert-butylamine and phenethylamine; the weak oxidant compound is selected from one of phosphite, organic peroxyacid and copper oxide. The temperature of the catalytic reaction is 35°C; the pH of the catalytic reaction is 6.7. When the two catalytic reaction steps are carried out simultaneously, the catalytic reaction time is 20h. The concentration of substrate glucose in the reaction system is 8g / L; the concentration of glucose isomerase in the reaction system is 4U / mL; the concentration of transaminase is 4U / mL.
[0046] The concentration of the amino donor compound in the reaction system was 1 mM; the concentration of the weak oxidant compound was 8 mM. The reaction system contains cof...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com