Ophthalmic preparation for preventing and treating dry maculopathy and retina light damage through eye drop administration
An ophthalmic preparation and preparation technology, applied in the field of ophthalmic drugs, can solve the problems of difficult to achieve effective treatment of ocular fundus diseases, difficult to enter the therapeutic concentration of the posterior segment of the eye, difficult to balance safety and effectiveness, etc., to avoid systemic side effects and properties. The effect of stabilizing and increasing medical compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1, the preparation of ophthalmic preparation of the present invention
[0088] Preparation method: Weigh 60mg of CMC-Na (sodium carboxymethylcellulose, ionic polymer) according to Table 1 and add it into a glass conical flask containing 10mL of pure water, turn on magnetic stirring for 2 hours to obtain solution 1; Add 0.3g of polysorbate 80 (surfactant) and 0.12g of HPC (low-substituted hydroxypropyl cellulose, thickener) into a glass conical flask containing 20mL of purified water, turn on magnetic stirring, and heat in a water bath at about 40°C for 1.5 hour, to obtain solution 2; weigh 15 mg of metformin hydrochloride and put it into solution 2, continue heating and stirring for 30 minutes, add solution 1, and stir for 30 minutes to obtain a mixed solution; disperse the mixed solution at a speed of 9500 for 5 minutes with a disperser, and stop the machine for 5 minutes. After the foam disappears, use a Buchner funnel to filter under reduced pressure to ob...
Embodiment 2
[0091] Embodiment 2, the preparation of ophthalmic preparation of the present invention
[0092] The preparation method refers to Example 1, and the raw materials and dosage are shown in Table 1, and a colorless and clear solution after removal of impurities is obtained.
[0093] pH and osmotic pressure adjustment: adjust to pH 6.5 with 1N sodium citrate solution, add sodium chloride to adjust osmotic pressure to: 297mOsmol / kg.
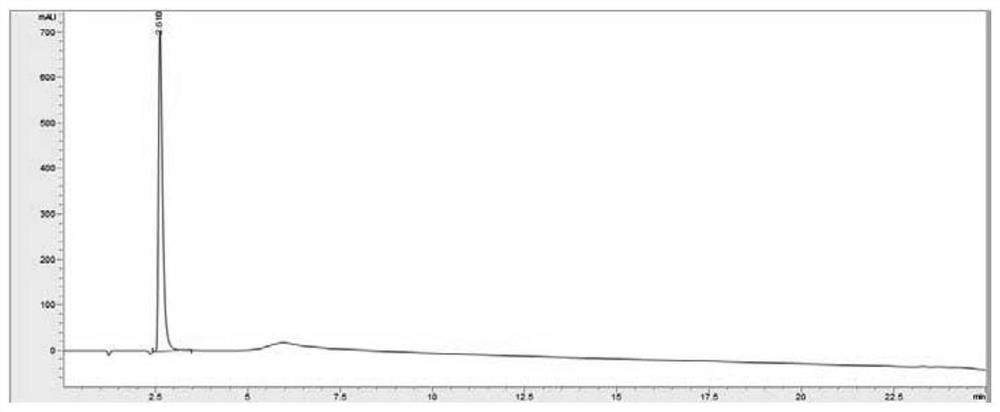
[0094] HPLC detection: Column: ZORBAX 300SB-CN, 2.1x150mm, 5μm; mobile phase: 40mM KH 2 PO 4 (pH4.5): Methanol (75:25) isocratic elution, Temp.: 35°C, detection wavelength: 233nm, Flowrate: 0.8ml / min; detection result: 99.1%.
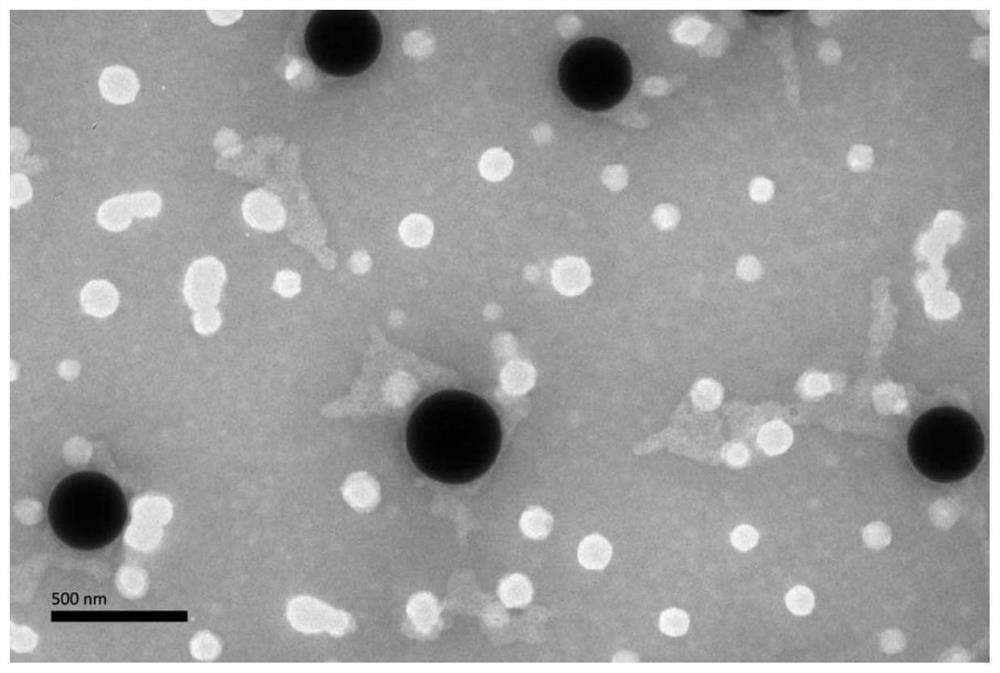
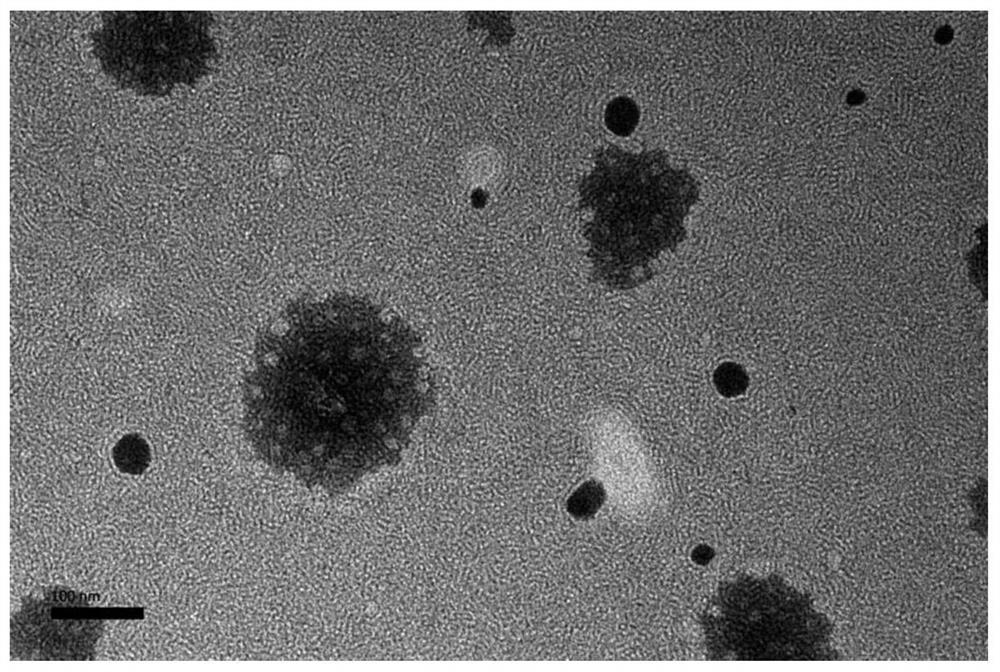
[0095] The particle size is 21.6nm (94.4%), PdI: 0.206; the appearance and content have no obvious change after being stored at room temperature in the dark for 1 month.
[0096] The concentration of API in the vitreous body of rats 1 hour after eye drops was: 39.8±16.6ng / g.
Embodiment 3
[0097] Embodiment 3, the preparation of ophthalmic preparation of the present invention,
[0098] The preparation method refers to Example 1, and the raw materials and dosage are shown in Table 1, and a colorless and clear solution after removal of impurities is obtained. pH test result: 6.9, close to isotonic, no need to adjust.
[0099] The HPLC detection method is the same as in Example 1, and the detection result: 98.6%.
[0100] The particle size is 16.6nm (98.6%), PdI: 0.227, and the appearance and content have no obvious change after being stored in the dark at room temperature for 1 month.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com