Method for preparing fluorine removal agent and treating fluorine-containing waste liquid
A technology of waste liquid treatment and fluoride removal agent, which is applied in chemical instruments and methods, water/sludge/sewage treatment, flocculation/sedimentation water/sewage treatment, etc. High cost and other problems, to achieve the effect of high fluoride removal efficiency, accelerated formation, and low operating cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
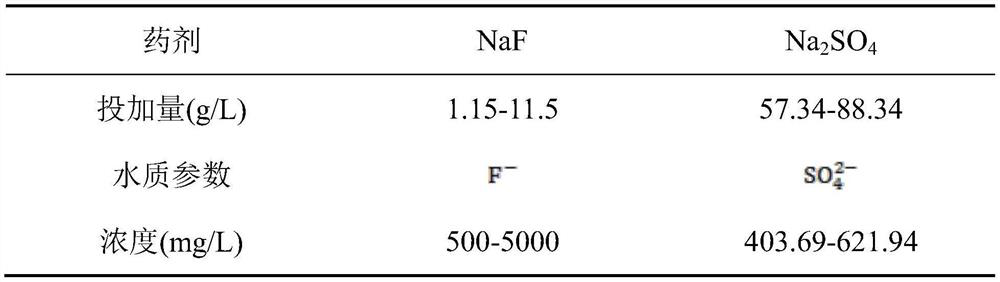
[0048] Used fluorine-containing waste liquid fluorine ion content 564mg / L in this example, sulfate radical content 606mg / L, pH=4.3, the preparation of modified magnesium iron defluoridation agent and fluorine-containing waste liquid treatment comprise the following steps:
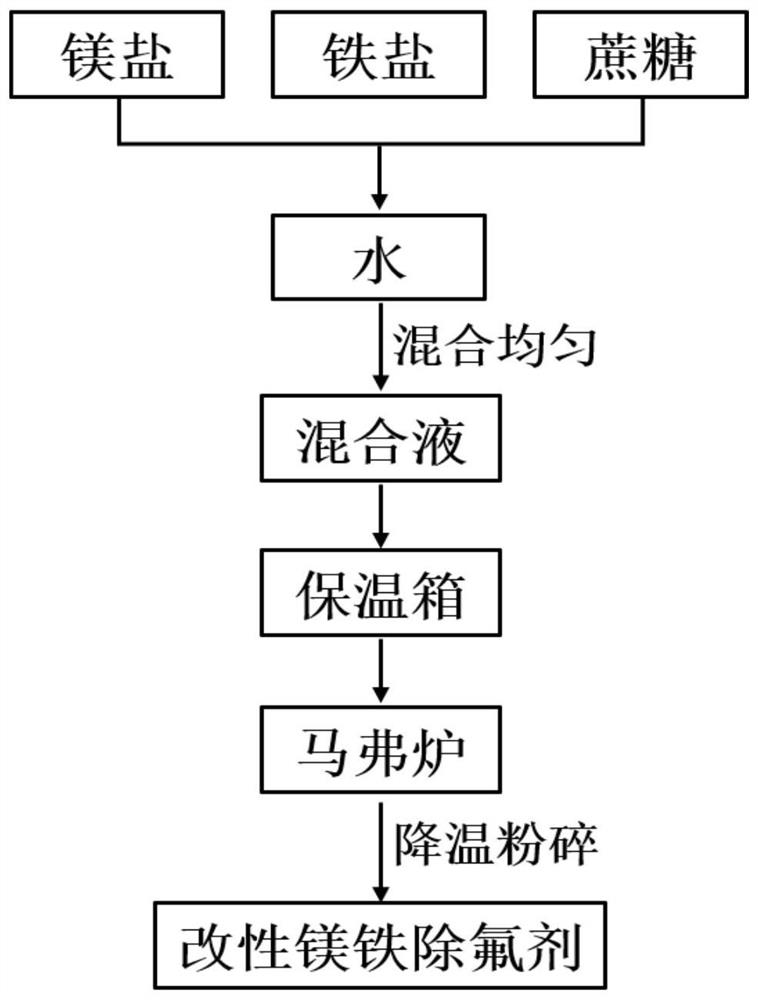
[0049] (17) Weigh MgCl 2 , MgSO 4 , FeCl 3 , Fe 2 (SO4) 3 , sucrose were respectively 3.6g, 4.4g, 2g, 5g, 6g, which were successively added to the beaker and dissolved with deionized water; placed on a magnetic stirrer and stirred for 1.5h;
[0050] (18) put into 105 ℃ incubator and dry;
[0051] (19) put into 400 ℃ muffle furnace for 2h;
[0052] (20) Take out and cool to room temperature, pulverize to obtain modified magnesium iron defluorination agent;
[0053] (21) Configure 1L of fluorine-containing waste liquid in a 2L beaker, detect the pH value of high-fluorine waste water, add hydrochloric acid, and control the pH value of the waste water to 4.3;
[0054] (22) Use the fluoride ion selective ...
Embodiment 2
[0058] The fluorine-containing waste liquid used in this example has a fluorine ion content of 611mg / L, a sulfate radical content of 588mg / L, and a pH=4.1. The preparation of the modified magnesium iron defluorinating agent and the treatment of the fluorine-containing waste liquid comprise the following steps:
[0059] (1) Weigh MgCl 2 , MgSO 4 , FeCl 3 , Fe 2 (SO4) 3 , sucrose were respectively 1.8g, 2.2g, 1g, 2.5g, 3g, which were successively added to the beaker and dissolved with deionized water; placed on a magnetic stirrer and stirred for 1h;
[0060] (2) put into 115 ℃ of vacuum ovens and dry;
[0061] (3) put into 430 ℃ muffle furnace for 2h;
[0062] (4) Take out and cool to room temperature, pulverize to obtain modified magnesium iron defluorination agent;
[0063] (5) Configure 1L of fluorine-containing waste liquid in a 2L beaker, detect the pH value of high-fluorine waste water, add hydrochloric acid, and control the pH value of the waste water to 4.1;
[00...
Embodiment 3
[0068] The fluorine-containing waste liquid used in this example has a fluoride ion content of 2678mg / L, a sulfate radical content of 602mg / L, and pH=4.4. The preparation of the modified magnesium iron defluorinating agent and the treatment of the fluorine-containing waste liquid comprise the following steps:
[0069] (1) Weigh MgCl 2 , MgSO 4 , FeCl 3 , Fe 2 (SO4) 3 , sucrose were respectively 1.8g, 2.2g, 1g, 2.5g, 3g, which were successively added to the beaker and dissolved with deionized water; placed on a magnetic stirrer and stirred for 1h;
[0070] (2) put into 120 ℃ incubator and dry;
[0071] (3) put into 400 ℃ muffle furnace for 3h;
[0072] (4) Take out and cool to room temperature, pulverize to obtain modified magnesium iron defluorination agent;
[0073] (5) Configure 1L of fluorine-containing waste liquid in a 2L beaker, detect the pH value of high-fluorine waste water, add hydrochloric acid, and control the pH value of the waste water to 4.4;
[0074] (6)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com